COA 2022 PBM Dirty Tricks Exposé Report
Executive Summary
There is growing awareness of the problems and pitfalls with Pharmacy Benefit Managers (PBMs) in the United States health care system. Contracted by plan sponsors (including government programs, self-insured employers, and insurance companies) to negotiate on their behalf with pharmaceutical companies, these “middlemen” corporations have quietly become an unavoidable part of our nation’s health care system.
Today, fewer than five PBMs control more than 80 percent of drug benefits for over 260 million Americans, which includes the power to negotiate drug costs, what drugs will be included on plan formularies, and how those drugs are dispensed. Oftentimes, patients are required to receive drugs through PBM-owned or affiliated specialty and mail-order pharmacies and suffer serious, sometimes dangerous, and even deadly, impacts of their abuses as a result of medication delays and denials.
However, while the role PBMs play in the U.S. health care system is complex and under scrutiny by both federal and state policymakers and the public, it is increasingly becoming clear that PBMs make up an oligopoly of rich, vertically integrated conglomerates that routinely prey on health care practices, providers, and their patients. PBMs have done this by overwhelmingly abusing their responsibility to protect Americans from this country’s drug pricing crisis, instead of exploiting the opacity throughout the nation’s drug supply chain to enrich themselves.
Unfortunately, their impact is only becoming more pronounced, especially in the world of cancer care. More and more cancer medications are coming out in oral formulations, resulting in a shift away from the medical benefit and into the pharmacy benefit. And because cancer medications are among the most expensive out there, they are very attractive to PBMs because they yield higher rebates, higher “DIR fees,” and other pricing gimmicks that yield substantial profits.
Through vertical integration and sheer market power, PBMs have also been able to creep into other areas of our health care system, such as injectable biosimilars and intravenous chemotherapies. Not only can PBMs leverage these products for steep originator drug rebates (thereby stifling the biosimilar industry for their own gain), but PBMs have also begun to institute policies such as mandatory “white bagging” to take the in-office administration out of the hands of patients’ oncologists.
The purpose of this exposé is to reveal and explain PBMs’ advantage and leverage by providing transparency where now there is total darkness, and by delving into the many ways that PBMs have abused their power. This report comprehensively explores and documents the myriad of PBM abuses, and their impact on patient care – focusing especially on cancer care. It explores how the recent levels of consolidation among PBMs and health insurers are adversely impacting cancer care, fueling drug costs, all while allowing for massive profits for PBMs and health insurance companies. Examining the most pervasive and abusive PBM tactics, each section highlights the adverse impact of PBMs on patients, healthcare payers (including Medicare, Medicaid, employers, and taxpayers), and providers, while also detailing potential solutions.
Each day that goes by, physicians, practices, and most importantly, patients become increasingly powerless because of horizonal PBM consolidation and vertical integration with insurers. The result is a system designed for patients to receive inferior treatment, while paying more out-of-pocket for their medications.
The time for sitting back and hoping for PBMs to become good faith actors is over. It is time for action to stop PBM abuses once and for all, and this exposé provides a road map for tackling them one dirty PBM trick at a time.
Introduction
In the eyes of many Americans, the problem with drug pricing is caused by unscrupulous pharmaceutical manufacturers who have increased drug prices over the last two decades with reckless abandon. This has been exemplified by a handful of highly visible bad actors, such as “pharma-bro” Martin Shkreli or Nostrum Pharmaceuticals founder, Nirmal Muyle, who rightfully captured the public’s attention, but wrongfully over-simplified the causes of our nation’s drug pricing issues.
Far more dangerous and insidious actors have quietly grown to dominate the nation’s pharmaceutical industry and drive high drug prices through the secretive pharmacy benefit manager (PBM) industry. Ironically, in the country’s attempt to rein in ruthless operators like Shkreli and Muyle, we ended up inadvertently creating the PBM problem that now plagues us. Expanding the role of PBMs, first from simple processors of pharmacy claims to middlemen more actively managing the prescription benefit initially made some sense. Clients – employers, unions, state governments, and other payers of medical care – did not have the expertise to manage complex drug benefits. Thus, they could hire a PBM to administer their prescription benefit, which would include simplifying and streamlining a complicated drug supply chain, designing formularies to exclude wasteful drugs, using their size and leverage to negotiate better discounts from pharmaceutical manufacturers, and managing pharmacy networks to create better outcomes for patients.
However, as this exposé on PBM business tactics, dirty tricks, and their negative impacts will detail, what seemed like a good idea “on paper” has not come to fruition. Instead, the nation’s largest PBMs have capitalized on the complexity of the drug supply chain and used the secrecy in which they operate to hide the true cost of drugs. And rather than eliminate the costly arbitrage within the supply chain, PBMs co-opted and embraced it, exacerbating the very problems of high drug prices that they were originally hired to control. They saw the financial windfall that would come through vertical integration and bought or set up their own mail-order and specialty pharmacies, steering patients away from independent community pharmacies and medical practices to their wholly-owned or affiliated pharmacy facilities where they could retain the inflated prices (and profits) they themselves were responsible for creating.
The perverse result is that PBMs have abandoned their most sacrosanct function of protecting their clients from high cost or low benefit drugs, instead letting higher priced drugs “buy” their way onto their clients’ formularies via rebates that the PBMs mostly retain. They then set up affiliated rebate aggregator entities to further obfuscate the flow of pharmaceutical manufacturer dollars, retaining a larger portion of their clients’ rebates, and leaving patients on high deductible plans exposed to drugs with exploitative list prices. The result is that patients pay more for their drugs off of artificially inflated list prices and the PBM clients have higher prescription drug costs.
The PBM’s purpose in the drug supply chain was to “police” the system. Had the largest PBMs not been lured in by the immense profit potential borne out of the complete opacity of drug costs, a PBM’s greatest asset would have been trust – trust from payers and providers that they were tirelessly working to protect the American public from high drug prices. However, this unfortunately did not come to pass. Instead, the PBM’s greatest advantage has become the almost total opacity of the U.S. drug supply chain and a lack of understanding among employers, unions, state governments, and American taxpayers of how most PBMs have chosen to abuse it.
The purpose of this exposé is to reveal and explain the PBM advantage by providing transparency where now there is total darkness and delving into the many ways that PBMs have abused their power to become “crooked cops.” Throughout this exposé, we comprehensively explore and document the myriad of PBM abuses, and their impact on patient care – focusing especially on cancer care. Finally, we explore how the recent levels of consolidation among PBMs and health insurers is adversely impacting cancer care, fueling drug costs, while allowing for massive profits for PBM and health insurance companies. We have thoroughly examined and detailed the most pervasive and abusive PBM tactics, in each section highlighting their adverse impact on patients, health care payers (including Medicare, Medicaid, employers and taxpayers), and providers.
With the ultimate goal of this exposé being transparency, Frier Levitt went beyond the law, partnering with 3 Axis Advisors LLC to create infographics derived from their analysis of millions of prescription claims across multiples states. The goal of these infographics is to help crystallize and simplify the very complex topics we will discuss throughout this exposé. Lastly, because PBMs have been known to hold themselves out as being “above the law,” we have provided the applicable law and legal principles governing each topic, and detailed the PBMs’ thin legal footing as it comes to these abusive practices. Finally, we have laid out potential, workable solutions to these issues, which may be legislative, regulatory, or legal in nature.
We intend for this report to serve as an authoritative source and reference guide for federal and state policymakers, regulators, and employers seeking greater understanding of PBM behavior, as well as frameworks for reshaping the industry for the better. While not all PBMs engage in these types of practices, or the degree with which they engage in these practices may vary from plan to plan, program to program, state to state, and so on, we believe that a thorough exposure of the blind spots, latitude for abuse, and backwards incentives is essential for any coherent understanding of the inherent flaws within the drug supply chain.
This exposé was commissioned by the Community Oncology Alliance (COA). The findings reflect the independent research of the authors, Frier Levitt, LLC, and does not endorse any product or organization. If this exposé is reproduced, we ask that it be reproduced in its entirety, as pieces taken out of context can be misleading.
Background
3.1 The Stakeholders
Any examination of the PBM industry must necessarily begin with an overview of the relevant stakeholders. These include five major categories of industry participants: (1) plan sponsors, (2) health insurers, (3) patients, (4) manufacturers, (5) providers, and (6) PBMs. Understanding who the major stakeholders are, and their relationship with one another, is paramount.
At the top of the hierarchy are plan sponsors. These include governmental health benefits programs (such as Medicare, Medicaid and TRICARE), employer-sponsored health plans, Taft-Hartley and union welfare plans, and private health insurance companies. These entities sponsor a health benefits plan for their members, beneficiaries or employees, and provide coverage for pharmacy expenses and drug costs (in addition to traditional medical expenses). In the Medicare Part D context, the Centers for Medicare & Medicaid Services (CMS) contracts with private insurance companies that submit bids to become Part D plan sponsors, and CMS in turn subsidizes certain costs associated with the operation of the plans. Likewise, in the Medicaid space, the majority of states operate a managed care model with respect to pharmacy benefits, contracting with Medicaid Managed Care Organizations (MCOs), who in turn, contract with PBMs to administer the pharmacy benefit. Finally, in the private sector, employers either directly or through an insurance company contract with PBMs to administer pharmacy benefits. These employer-sponsored plans may either be fully-insured (meaning the employer hires an insurance company and pays all or part of the premiums on behalf of its employees) or self-insured (meaning the employer bears all of the financial risk with the costs of care). In any case, these plan sponsors bear the ultimate costs of care, and suffer when PBM abuses cause prices to rise or waste to occur. Plan sponsors may or may not hire a health insurance company to help offset the risks associated with the cost of care, and pay premiums on behalf of their beneficiaries. These health insurance companies may in turn be the entity that directly contracts with the PBM for pharmacy care. However, as noted below, the lines have become increasingly blurred between health insurers and PBMs; thus, the key distinction between plan sponsors and health insurers is that the plan sponsors are typically the ultimate financial guarantors of the costs of the health care for their beneficiaries, including not only drug costs but also major medical expenses.
At the other end of the continuum are the patients. Patients include beneficiaries of government sponsored health care programs, as well as the employees (and dependents) of employers sponsoring health plans. They are also uninsured or underinsured individuals who are left to find a way to cover drug costs themselves. In oncology, they are cancer patients needing care from a complex and disjointed health care system. As a group, they not only bear a disproportionate share of the out-of-pocket costs associated with PBM abuses, but also suffer from the inferior care caused by certain PBMs’ tactics of putting profits over patients. These include delays and denials as a result of PBMs’ unnecessary obstacles to care.
On the front line of care are the providers. These include retail, specialty and mail-order pharmacies, and in oncology, community oncology practices. In addition to providing direct medical care, community oncology practices provide in-office and outpatient pharmacy services, which can take two basic forms (depending on applicable state law): dispensing physician practices (i.e., in-office dispensing under a plenary medical license), or oncologist-owned pharmacies (i.e., the oncology practice owns and operates a licensed retail pharmacy within the clinic). These providers contract with PBMs to dispense medication to plan members, and participate in PBM networks. In so doing, they are tasked with providing appropriate care to their patients, while remaining bound to the PBMs who set reimbursement rates and other terms for participation.
While not directly involved in the provision of care, manufacturers are equally part of the continuum and impacted by PBM actions. These include drug and biologic manufacturers, including both brand and generic companies. Manufacturers have had a particular important role in the biosimilar market, becoming captive to PBMs’ rebate traps, and stifling the biosimilar market before it even has a chance to take hold.
The final piece of the puzzle is the PBM. PBMs are third-party administrators of prescription drug programs covered by a plan sponsor. The PBM is primarily responsible for processing and paying prescription drug claims submitted by participating providers on behalf of covered beneficiaries. However, a PBM’s role is not limited to processing and paying prescription drug claims. Rather, PBMs also provide bundled services related to the administration of pharmaceutical benefits, including formulary design, formulary management, negotiation of branded drug rebates, and controlling network access of participating pharmacies. Perhaps most importantly, PBMs often also own and operate their affiliated retail, mail-order and/or specialty pharmacies, and in so doing, directly compete with independent providers participating in PBM networks. They are not just the gatekeepers, but also competitors operating in the same marketplace. This blatant conflict of interest has serious consequences. Finally, as the result of consolidation and vertical integration within the marketplace, virtually all of the major PBMs have merged with, acquired or become acquired by health insurers, greatly blurring the lines between insurer and PBM. As a result, health insurers and PBMs are often referred to jointly as “payers.”
Figure 1. The Pharmacy Benefits Landscape
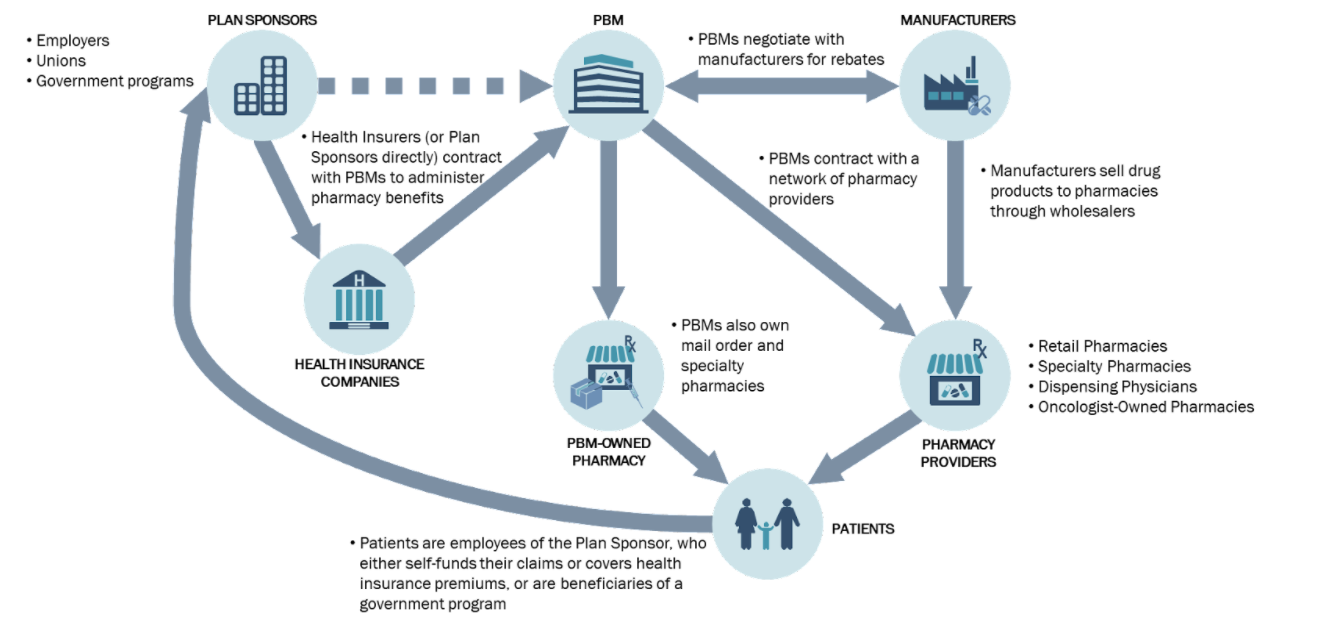
The Figure 1, above, visually demonstrates the different stakeholders, and their relationship with one another.
3.2 Consolidation of PBMs and Health Insurers, and the Resulting Influence on Recent PBM Actions
PBMs traditionally have played a critical role in the administration of prescription drug programs. However, over the past ten years, the PBM marketplace has transformed considerably. Changes include both horizontal and vertical integration among health insurance companies, PBMs, chain pharmacies, specialty pharmacies, and long-term care pharmacies. As a result, a smaller number of large companies now wield nearly limitless power and influence over the prescription drug market.
Within the PBM marketplace, over 80% of the covered lives in the United States are controlled by only five PBMs. As a result of this concentration, a pharmacy’s access to these five PBM networks is critical. Being out of network with just one PBM (which in some regions, could make up more than 85% of the market), and being unable to obtain reimbursement for claims dispensed to those patients, could make it financially unviable for any community oncology practice to provide dispensing services at all. The lack of competition in the marketplace stems, in large part, from a series of mergers, integrations, and consolidations. These consolidations and integrations are undoubtedly a factor in many abusive PBM practices, ranging from seeking to exclude independent providers, to reimbursement rates that force providers to lose money by filling prescriptions, to outright diversion of patients to the PBMs’ wholly-owned or affiliated pharmacies. The consolidation increases the market power of the top PBMs, which makes this possible.
The breadth of PBM power did not arise overnight. It began with a series of vertical consolidations in which some PBMs acquired pharmacies and other PBMs acquired insurance companies. In 2007, the shareholders of Caremark Rx, one of the nation’s largest PBMs at the time, approved a $26.5 billion takeover of CVS Pharmacy, which effectively created the first vertically integrated retail pharmacy and PBM. Vertical integration of the industry continued in 2011, as Blue Cross Blue Shield of North Carolina, one of Medco’s largest customers, began shifting its PBM business away from Medco to Prime Therapeutics, a PBM that is wholly owned by a group of thirteen Blue Cross plans across the country. In 2012, UnitedHealthcare (United), the nation’s largest insurance company, began migrating the administration of its plans from Medco Health Solutions to OptumRx, United’s wholly-owned PBM.
Consolidation of the PBM and payer space has not been limited to vertical integration. In 2011, two of the nation’s then-largest PBMs – Medco Health Solutions, Inc. and Express Scripts, Inc. – announced a $29 billion merger. After a contentious regulatory approval process, the Federal Trade Commission ultimately approved the merger in 2012.
Thereafter, the industry continued consolidation both horizontally and vertically. In 2013, a regional PBM – SXC Corporation – agreed to buy another regional PBM – Catalyst, Inc. – for $4.4 billion to form a national PBM, known as Catamaran Corp. In July 2015, Catamaran was acquired by United, OptumRx’s parent company, for $12.8 billion. The two PBMs are now integrating operations and operate under one name, OptumRx. In 2015, Rite Aid acquired the PBM EnvisionRx for approximately $2 billion. Later that year, Walgreens announced its intention to acquire Rite Aid and EnvisionRx for $9.4 billion. Also in 2015, Aetna, the nation’s third-largest insurer, announced its intention to acquire Humana, the nation’s fourth-largest insurer, as well as Humana’s wholly-owned PBM, Humana Pharmacy Solutions, for $37 billion. Finally, in 2015, Anthem announced its agreement to buy Cigna (including its PBM arm) for $48 billion, which would result in, yet again, fewer players in the space. However, on July 21, 2016, the Justice Department filed lawsuits to block both the Aetna-Humana and Anthem-Cigna mergers, asserting that the mergers would quash competition, leading to higher prices and reduced benefits.
Figure 2. PBM Mergers and Consolidations in Last Ten Years

Unfortunately, the last five years has only seen this trend of consolidation and integration expand at an exponential rate. In November 2018, CVS Health completed a controversial $69 billion acquisition of Aetna, a managed health care company that specializes in selling traditional and consumer-directed health insurance along with related services including dental, vision, and disability plans. Not to be outdone, in December 2018, health insurer Cigna acquired Express Scripts for $54 billion. Since that time, Cigna and Express Scripts have continued to expand in creative ways. In December 2019. Express Scripts and Prime Therapeutics announced a three-year collaboration agreement, whereby Express Scripts would take over the contracting and administration of the pharmacy benefits for Prime Therapeutics’ members. As a result of the arrangement, Express Scripts will now manage the prescription benefits for more than 100 million Americans.
Figure 3. Vertical Integration of PBMs and Health care Conglomerates
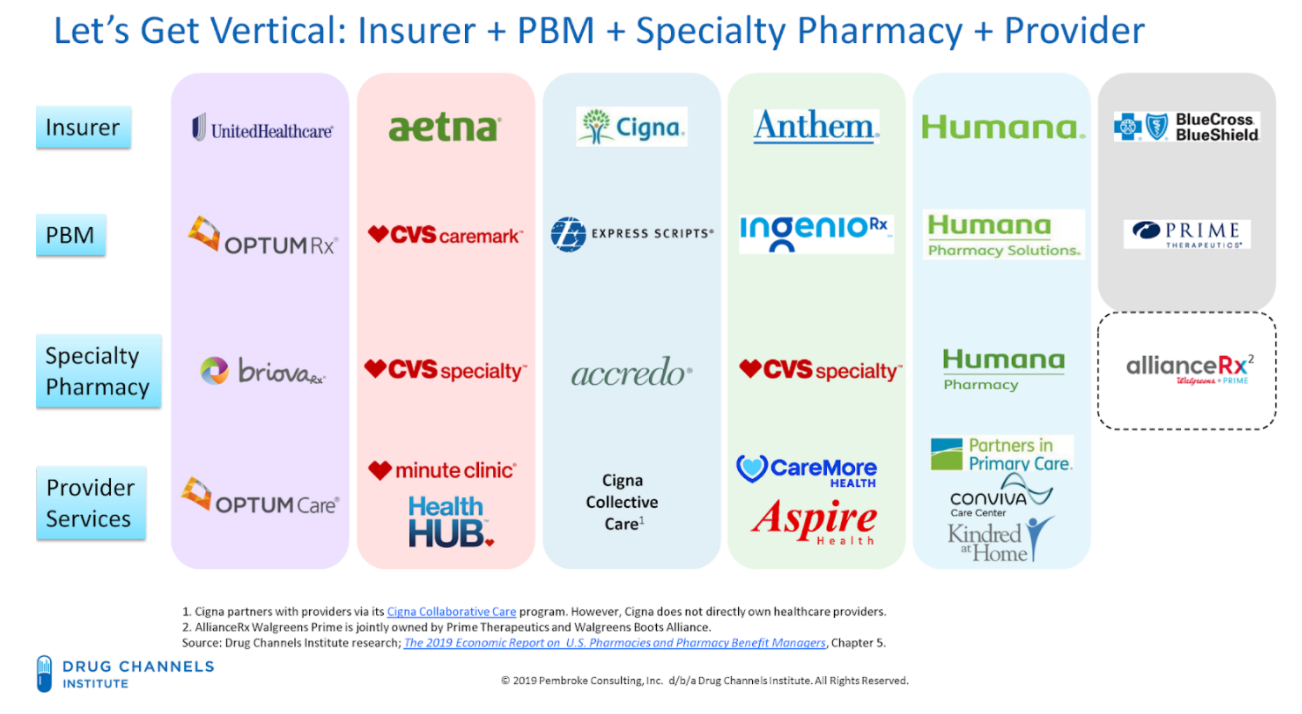
This rapid evolution of the PBM and health insurance industry shows how a limited number of corporations wield an outsized level of control and influence in the prescription drug coverage marketplace. Fewer payers spells harm to patients, especially cancer patients. These integrated companies have greater abilities to control the nature and direction of patients’ care, including what type of care/drugs they receive, from whom they receive it, and in what setting they are treated. The level of PBM intrusion into the care received by patients borders on the practice of medicine by these PBMs and health insurance conglomerates.
Fewer payers also results in harm to plan sponsors, especially employers sponsoring health plans, who have fewer choices based on decreased competition. This hits small employers the hardest, who lack the overall leverage and resources to either demand competitive rebates or restructure entrenched PBM practices.
Fewer payers also exponentially increases the importance of network access for providers. Exclusion from one PBM with a market share of 35% means that the provider loses out on a major portion of the patient population.
Figure 4. Market Share by PBM in U.S. Prescription Benefits Market in 2018
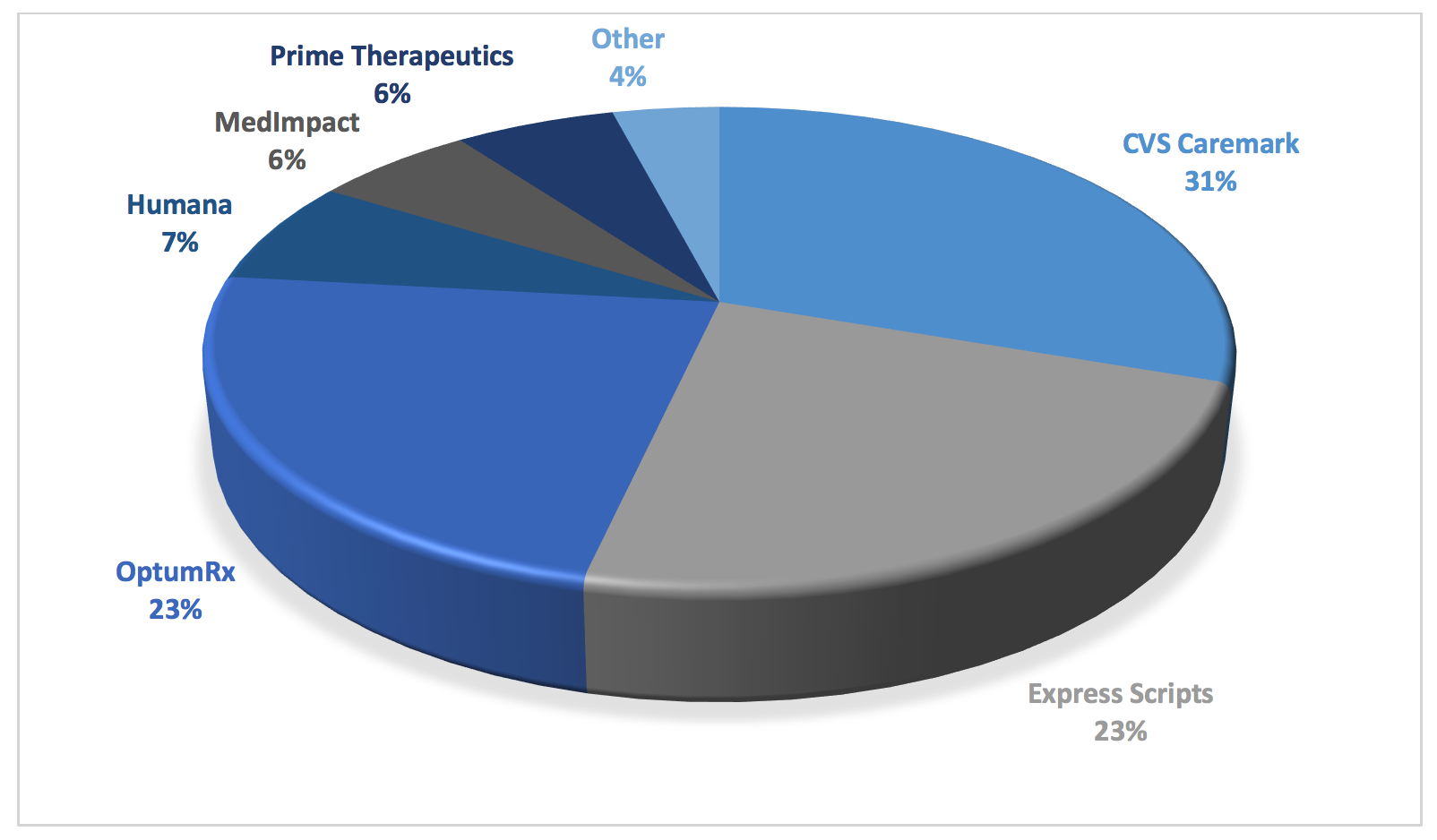
As can be seen in the figure above, consolidation has created merged entities that have oppressive power. This creates a virtual chokehold note only on community oncology practices and pharmacy providers, but on plan sponsors and patients alike. It is through this market dominance that PBMs are able to get away with their abuses. Whether it is outsized rebates and DIR fees fueling drug prices. Whether it is unreasonable barriers to entry, network exclusions, or mandatory white bagging forcing patients to receive inferior service at higher costs. Whether it is employing insidious copay accumulator programs or deceptive pricing and reimbursement techniques. Or worse yet, whether it is essentially practicing medicine, through “fail first” step therapy, prior authorization requirements, or formulary exclusions, many of which favor not the least expensive medication, but the most profitable one for the PBM. Each of these tactics are made possible by the PBMs’ sheer levels of dominance at all levels of the health care continuum. This consolidation has hurt medical care while fueling both drug prices and costs to patients and plan sponsors alike.
While the Federal Trade Commission (FTC) and Department of Justice (DOJ) Antitrust Division recently embarked on a process to rewrite vertical merger guidelines, this effort is seen by many as coming “too little, too late.” Providers, patients, and plan sponsors have long realized that the vertical integration between payer-PBM-provider would spell disaster for quality and freedom of choice. Dramatic and urgent action is necessary to curtail this wide-ranging abuse of power.
Manufacturer Rebates, Rebate Aggregators, and the “Gross-to-Net Bubble”
It is axiomatic to say that the PBM market is highly concentrated, with three companies (i.e., CVS Caremark, Express Scripts, and OptumRx) covering nearly 80 percent of the market or 180 million American lives. As a result, pharmaceutical and biosimilar manufacturers face exceedingly high stakes when negotiating for formulary placement. Among the different sources of revenue, the most prolific by far is in the form of rebates from pharmaceutical manufacturers that PBMs extract in exchange for placing the manufacturer’s product drug on a plan sponsor’s formulary or encouraging utilization of the manufacturer’s drugs. Rebates are mostly used for high-cost brand-name prescription drugs where there are interchangeable products and aim to incentivize PBMs to include pharmaceutical manufacturers’ drugs on plan sponsors’ formularies and to obtain preferred tier placement.
While drug prices are too high, ironically, the growing number and scale of rebates is the primary fuel of today’s high drug prices. The truth is that PBMs have a vested interest to have drug prices remain high and to extract rebates off of these higher prices. PBM formularies tend to favor drugs that offer higher rebates over similar drugs with lower net costs and lower rebates.
Figure 5. Gross-to-Net Bubble
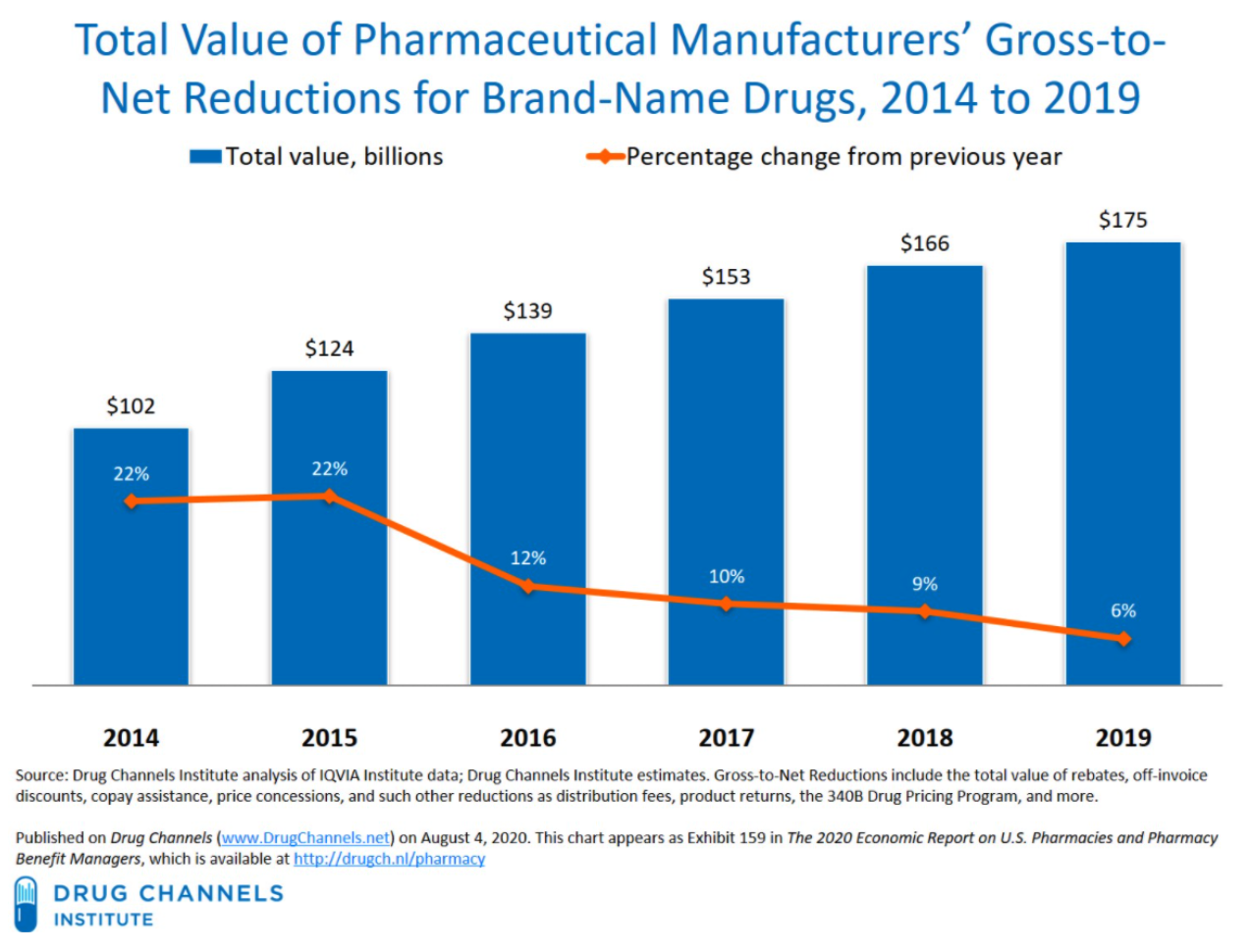
Apart from increasing costs today, these destructive practices will have a long-lasting impact on the future of health care and drug innovation. Traditionally, generic drugs offer significant price relief for brand medications; however, there are an ever-growing subset of medications that are unlikely to ever have a traditional generic alternative. As a result, federal policy was enacted to create eventual competition for these brand products such as the biosimilar pathway. However, the PBMs’ practice of maximizing rebates may effectively neuter the nation’s biosimilar market before it even gets off the ground. Unlike traditional drug products, biologics are unique and complex molecules and represent many of the new breakthrough treatments that have come to market over the past ten years. But with such breakthrough comes extremely high cost. As a result, biosimilars – that is, products that are “highly similar” to the reference biologic – have emerged to provide alternatives and competition in the biologics space. The first biosimilar product in the United States was approved in March 2015 and marketed in September 2015. The greater use of biosimilars has the potential to reduce the overall drug spending, while providing greater clinical options for providers and patients. However, PBMs and biologics manufacturers have erected “rebate walls” that have severely depressed biosimilar development and widespread adoption. According to former FDA Commissioner, Dr. Scott Gottlieb, Americans could have saved more than $4.5 billion in one year alone, if they had bought FDA-approved biosimilars. While the FDA had approved 11 biosimilars through 2018, only three were then being marketed in the U.S. As of January 2022, nearly 32 biosimilars have been approved, while only 29 are currently being marketed. PBM rebates represent a clear and existential threat to the future of the biosimilar marketplace.
As the American public and plan sponsors have become more aware of the nature and extent of rebates, they have begun demanding that all or nearly all rebates negotiated on their behalf be fully reported and passed-through. As a result, PBMs have begun to market themselves as transparent and assert that many of their customers are able to negotiate “pass-through pricing” allowing pharmaceutical manufacturer rebates and other concessions to flow directly to plan sponsors. However, a dangerous new trend has grown exponentially over the last few years through which PBMs seek to “circumvent” these pass-through requirements. PBMs have increasingly “delegated” the collection of manufacturer rebates to “rebate aggregators,” which are often owned by or affiliated with the PBMs, without seeking authorization from plan sponsors and without telling plan sponsors. Sometimes referred to as rebate GPOs, these mysterious entities include Ascent Health Services, a Switzerland-based GPO that Express Scripts launched in 2019, Zinc, a contracting entity launched by CVS Health in the summer of 2020, and Emisar Pharma Services, an Ireland-based entity recently rolled out by OptumRx. Even some of the major PBMs (i.e., the “Big Three” PBMs) sometimes find themselves contracting with other PBMs’ rebate aggregators for the collection of manufacturer rebates (for example, in the case of OptumRx contracting with Express Scripts for purposes of rebate aggregation for public employee plans).
In both the private sector and with respect to government health care programs, the contracts regarding manufacturer rebates (i.e., contracts between PBMs and rebate aggregators, as well as contracts between PBMs/rebate aggregators and pharmaceutical manufacturers) are not readily available to plan sponsors. Moreover, PBMs do not provide plan sponsors access to claim-level rebate information unless demanded through the contracts entered by and between plan sponsors and PBMs.
Within Medicare Part D, Part D Sponsors are required to submit Direct and Indirect Remuneration (DIR) reports to CMS disclosing the total amount of rebates, inclusive of manufacturer rebates, retained by PBMs regardless of whether such rebates were passed to Medicare Part D plan sponsors. And while PBMs and rebate aggregators are obligated to provide, among other things, the aggregate amount and type of rebates, discounts, or price concessions to the plan sponsors (who in turn provide the same to CMS), PBMs and rebate aggregators do not have to provide claims-level information on the actual amounts received on behalf of plan sponsors.
4.1 Who is Impacted?
The deleterious effects of rebates, and the furtive work of rebate aggregators, are felt across the health care spectrum.
4.1.1 Harm to Patients
Whether a patient has insurance or not, rebates serve to increase the overall costs of drugs and out-of-pocket expenditures for patients. With one in four people in the United States having difficulty paying the cost of their prescription medications, the extent of the negative impact of rebates is felt far and wide.
For uninsured patients, the rebates negotiated by a PBM or health insurance company do nothing to lower their out-of-pocket costs. Rebates promote high drug list prices. “Higher drug prices hurt uninsured patients who pay list prices … based on drugs’ list prices.” And because these rebates are received and kept among secretive health care conglomerates, and not shared with providers or other groups, even discount programs like GoodRx do little to help uninsured patients receive savings on the most expensive drugs.
Even for patients with insurance, rebates ultimately increase costs to the patient for the benefit of PBMs and health insurers. At the point of sale, the inflated list prices caused by rebates “hurt … insured patients who pay coinsurance and deductibles based on drugs’ list prices.” Over the past several years, the number of patients on high-deductible health plans has skyrocketed. This has turned the insurance market upside down, causing the relatively small number of sick patients who pay high copays off of inflated list prices to subsize the cost of care for healthy people. In this form of “reverse insurance,” the sickest patients (e.g., those taking expensive cancer medications) generate a large share of manufacturer rebate payments, which in turn are used to “subsidize the premiums for healthier [patients].” This is the opposite of how insurance is supposed to work.
What’s worse, PBMs’ preference of highly-rebated drugs not only increases patients’ out-of-pocket expenses, but also creates unnecessary burdens in receiving appropriate care, even to the point of fatality. PBMs have an incentive to favor high-priced drugs over drugs that are more cost-effective, because rebates are often calculated as a percentage of the manufacturer’s list price. PBMs receive a larger rebate for expensive drugs than they do for ones that may provide better value at lower cost. This can also occur “when a brand drug goes generic under the Hatch-Waxman Amendments, with the first generic version being granted six months of market exclusivity,” and “[i]n exchange for substantial rebates, manufacturers [are given] an exclusive extension of their brand drug, which circumvents Hatch-Waxman and blocks generic competition.” PBMs’ financial motivations often result in more expensive and less efficacious drugs being placed on the drug formulary, which in turn hurts patient care.
Again, PBMs are able to do this because of the sheer levels of market consolidation and integration, which is adversely impacting cancer care and fueling drug costs all in the interests of PBM profits.
4.1.2 Harm to Plan Sponsors
While rebates are intended to lower the “net price” of drugs, thereby reducing costs to plan sponsors (including employers), there are several important ways that PBM rebates increase the costs of drugs for both plan sponsors and patients.
The first way relates to the ability of plan sponsors, especially self-funded employers, to ensure the full amount of rebates are reported and passed through to them by PBMs. As noted above, it is extremely difficult to gauge the true amount of drug manufacturer rebates collected by PBMs, and this is only made more difficult by the advent of rebate aggregators. Unlike in the Medicare Part D program, PBMs typically do not legally owe self-funded employers any reporting on rebates. PBMs employ exceedingly vague and ambiguous contractual terms to recast monies received from manufacturers outside the traditional definition of rebates, which in most cases must be shared with plan sponsors. Rebate administration fees, bona fide service fees, and specialty pharmacy discounts/fees are all forms of money received by PBMs and rebate aggregators which may not be shared with (or even disclosed to) the plan sponsor. These charges serve to increase the overall costs of drugs, while providing no benefit whatsoever to plan sponsors.
And while there might be greater reporting and disclosure obligations in the Medicare Part D and Medicaid programs, the growth of rebate aggregators has created a way for PBMs (or their corporate affiliates) to retain rebates and not share them with plan sponsors. This causes the Part D plan sponsor to become liable to CMS to “true up” any reductions in cost caused by these rebates, despite the fact that the Part D plan sponsor never actually received any rebates. Moreover, studies have shown that PBM rebates extracted from drug manufacturers drive up the drug spending of plan sponsors including Medicare and Medicaid. This is especially draining on already budget-strapped state governments. Since Medicare Part D is financed through general revenues, beneficiary premiums, and state payments for dual-eligible beneficiaries (who received drug coverage under Medicaid prior to 2006), rebates also drive up the drug spending of the participating states and in turn, taxpayers’ financial obligations to support Medicare Part D and Medicaid continues to rise. The total drug spend of a plan sponsor, regardless of whether it is a federal or state governmental program or a self-funded employer, will inevitably increase because PBMs are incentivized to favor expensive drugs that yield high rebates. In some instances, PBMs purposely misclassify generic drugs as brand drugs to charge higher prices to plan sponsors, which ultimately generate higher rebate revenue. Moreover, the gross-to-net bubble (i.e., the dollar difference between sales at brand-name drugs’ list prices and their sales at net prices after rebates, discounts, and other reductions) has been growing at an exponential pace. The upward trend in the gross-to-net bubble reached $175 billion in 2019. Based on this trend and the fact that plan sponsors are not receiving full value of the rebates from PBMs, it is evident that rebates increase total drug spend of plan sponsors and only benefit PBMs.
The final and perhaps most long-term impact that rebates will have on plan sponsors is in the suppression of the biosimilar market. The greater use of less expensive biosimilars (essentially “generic” versions of biologic medications) has the potential to reduce overall drug spending. However, many health plans do not include biosimilars in their preferred tiers. This is because of the “rebate trap,” where PBMs prefer the higher cost, branded biologics that offer rebates, over cheaper biosimilar alternatives. The result is that when biosimilars do make their way to the market, many patients do not have access to them because their PBM does not cover it. These policies stifle advancements, and will, in the long term, keep plan sponsors beholden to higher cost, branded medications.
4.1.3 Harm to Providers
Finally, rebates also impact providers in several ways. First, PBMs preference of highly rebated drugs limits providers’ choice of optimal drug therapy for patients. Once again, this results in the PBM inserting itself in between the prescribers and their patients and violates the sanctity of the doctor-patient relationship. This is especially true with biosimilars. The greater use of biosimilars has the potential to reduce overall drug spending and provide greater clinical options for providers, including community oncology practices. However, due to rebates, many PBMs do not include biosimilars in their preferred tier, thereby preventing widespread adoption and cost savings.
In instances where biosimilars are included on formularies, this is done so inconsistently and on a patchwork basis, tied solely to the rebates that the PBM can extract from the drug manufacturer, and not the efficacy of the product. The result is that community oncology practices often are required to stock several different versions of very expensive biosimilars based on the rules of the patient’s PBM, rather than being able to prescribe and dispense the product that is best suited for their patients.
Rebates further intrude on the doctor-patient relationship when combined with step therapy, prior authorization, or other utilization management protocols. “Fail first” step therapy requires a patient to first fail once or twice on a medication specified by the PBM or health insurer before being allowed to “step up” to the therapy prescribed by the physician. In many cases, the medication dictated by the PBM or health insurer is not the least expensive medication, but rather, is the most profitable drug to the PBM due to rebates. The impact of step therapy, driven by rebating, is that it “takes the medical decision-making out of the hands of doctors” and puts it into the hands of the actuaries, accountants, and business people at the PBM, who are not choosing the drug that is most efficacious, or cheapest, or even most efficient – they are choosing the drug that is the most profitable.
4.2 What Does the Law Say?
Medicare Part D plan sponsors are required to submit DIR reports to CMS disclosing the total amount of rebates, inclusive of manufacturer rebates and pharmacy rebates, retained by PBMs regardless of whether such rebates were passed to Medicare Part D plan sponsors.
In the commercial market, many states have enacted laws that require transparency from PBMs and “pass through” pricing. For example, Delaware House Bill 194 enacted into law on July 17, 2019, permits the Insurance Commissioner to examine the affairs of PBMs, among other things. Likewise, under New York Senate Bill S1507A enacted into State Budget for the 2019-2020 Fiscal Year on April 12, 2019, PBMs are required to fully disclose to the Department of Health and plan sponsors the sources and amounts of all income, payments, and financial benefits. Similarly, Utah House Bill 272, which was enacted into law on March 30, 2020, requires PBMs to report all rebates and administrative fees to the Insurance Department including the “percentage of aggregate rebates” that PBMs retained under its agreement to provide pharmacy benefits management services to plan sponsors.
However, Maine Bill 1504, enacted into law on June 24, 2019, takes these reporting requirements a step further, and provides that “[a]ll compensation remitted by or on behalf of a pharmaceutical manufacturer, developer or labeler, directly or indirectly, to a carrier, or to a pharmacy benefits manager under contract with a carrier, related to its prescription drug benefits must be: A. Remitted directly to the covered person at the point of sale to reduce the out-of-pocket cost to the covered person associated with a particular prescription drug; or B. Remitted to, and retained by, the carrier. Compensation remitted to the carrier must be applied by the carrier in its plan design and in future plan years to offset the premium for covered persons.”
4.3 What Can Be Done?
If high drug prices meaningfully addressed then outsized negative impact of rebates, rebate aggregators, and the resulting high gross-to-net bubble must be addressed. Luckily there are several varied options available to the affected parties:
- Legislative
- Policymakers should enact laws that mandate PBMs and rebate aggregators to report drug manufacturer rebates procured by utilizing drugs dispensed to plan sponsors’ patients in a given year. Requirements set forth under 42 CFR § 423.514(d) are not sufficient to cast the light of full transparency on PBMs (and rebate aggregators) that contract with Medicare Part D plan sponsors.
- Laws should be enacted that allow plan sponsors to gain access to the drug manufacturer rebates reported by PBMs and rebate aggregators.
- Laws should be enacted that entitle Medicare Part D plan sponsors and state Medicaid agencies to conduct full and complete audits of PBMs and rebate aggregators and these entities should not have any ability to limit the scope and extent of such audits.
- Laws should be enacted that limit Medicare Part D plan sponsors’ financial obligation to CMS in the event that PBMs and rebate aggregators retained drug manufacturer rebates that were not relayed to Medicare Part D plan sponsors.
It should be called out that some in Congress have the mistaken belief that drug manufacturers are the primary beneficiary of rebates in terms of “buying” formulary access for their drugs. Although this may be true in a limited number of cases, the reality is that PBMs use rebates to extract – some would say “extort” – drug manufacturers to pay the rebate “toll” in order for PBMs to include these drugs on formulary or to avoid being part of a “fail first” step therapy scheme. Congress has been held hostage to PBMs and their corporate affiliated health insurers by threatening to increase plan premiums if rebates are eliminated or made illegal.
- Plan Sponsor Action
- As part of the PBM contracts, plan sponsors should:
- Require PBMs to seek approval from plan sponsors prior to delegating the rebate aggregation function to rebate aggregators.
- Require PBMs to disclose a list of rebate aggregators to plan sponsors.
- Require PBMs to disclose an unredacted contract with the rebate aggregator.
- Require PBMs to be pay fees to rebate aggregators for their services but such fees should not come from drug manufacturer rebates.
- Require PBMs to agree to rebate audits conducted by plan sponsors and/or third-party auditors at plan sponsors’ choosing.
- Require PBMs to report claims-level data on rebates collected on claims paid by pan sponsors.
- As part of the PBM contracts, plan sponsors should:
Pharmacy Direct and Indirect Remuneration Fees
As a result of a 2014 CMS rule change that went into effect in Plan Year 2016, PBMs have developed shrewd and calculated methods of financial engineering, maximizing their revenue at the expense of the patient, the Medicare Part D Program, and providers. This was accomplished through pharmacy direct and indirect remuneration fees, or “DIR fees.” DIR fees are typically post point-of-sale fees ranging from 1.5% to 11% of a drug’s list price assessed by PBMs upon network pharmacy providers, typically three to six months after the provider has dispensed the medication.
The concept of DIR fees arose out of Medicare Part D coverage for prescription drugs. Part D plan sponsors and Medicare Advantage plans offering drug coverage are paid by the government based on the actual cost for drug coverage. The actual cost is based on the Part D plan sponsor’s “negotiated price,” which is then used as the basis to determine plan, beneficiary, manufacturer (in the coverage gap), and government costs during the course of the payment year, subject to final reconciliation following the end of the coverage year.
Unfortunately, very few pharmacy price concessions have been included in the negotiated price at the point of sale. All pharmacy and other price concessions that are not included in the negotiated price must be reported to CMS as pharmacy DIR. As employers and plan sponsors are demanding a greater share of the PBM rebates, and as those rebates have been threatened with regulation by state and federal lawmakers, PBMs have gone “downstream” to make up for any rebate revenue shortfalls by assessing DIR fees on pharmacy providers. In fact, DIR fees categorized as pharmacy price concessions have increased 45,000 percent between 2010 and 2017, and have hit a whopping $9.1 billion in 2019.
Figure 6. Explosion of Pharmacy DIR from 2013 to Present
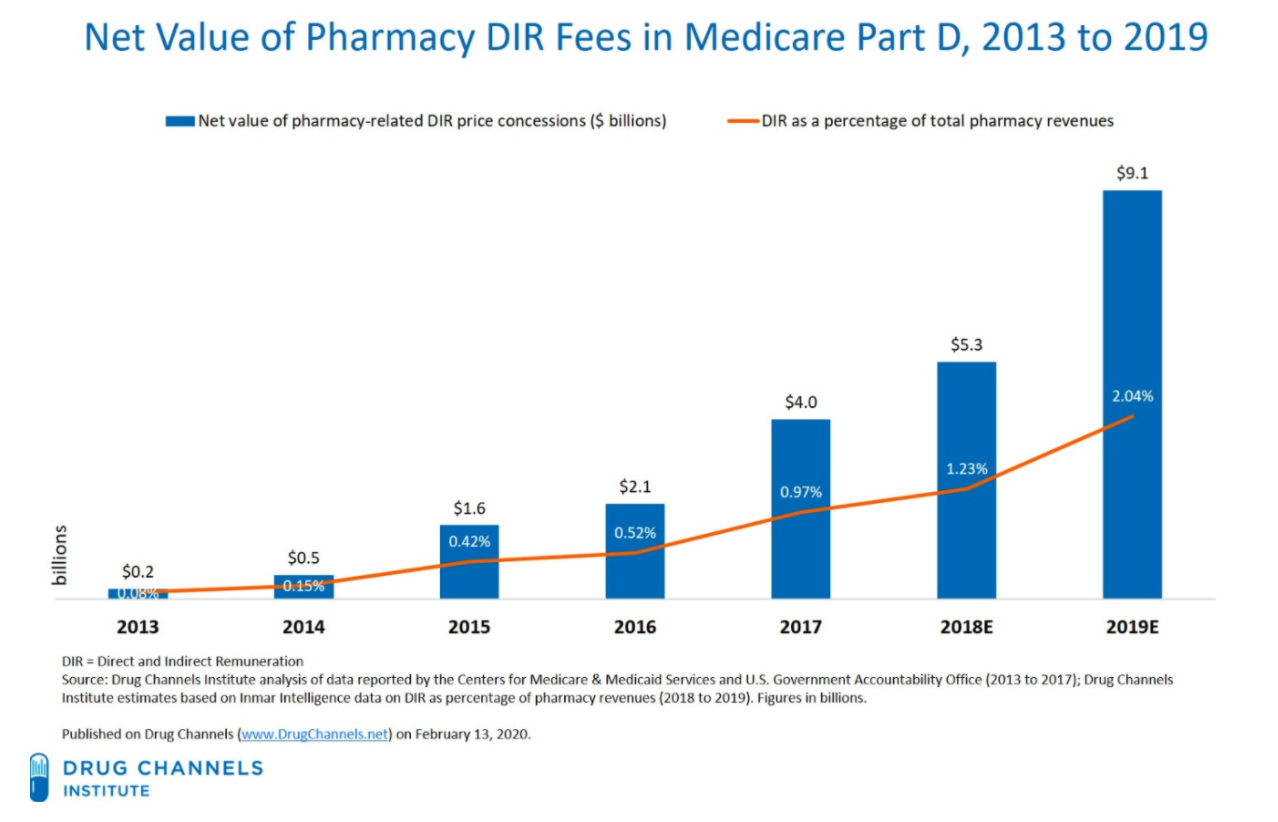
PBMs purport to pass a large portion of DIR fees to their plan sponsor clients, especially Part D plan sponsors – ironically, many of which are under the same corporation as the PBMs (e.g., CVS Caremark, one of the nation’s largest PBM, and SilverScript, the nation’s largest Medicare Part D plan sponsor, are both owned by CVS Health). However, no study has been conducted to match the deductions from pharmacy remittances for “DIR” with the DIR reported to CMS. Unfortunately, CMS cannot even perform such an audit today, as it does not require plans to submit DIR collected from each pharmacy, but rather requires DIR to be reported by drug, on an NDC number basis.
Even if pharmacy DIR fees are reported accurately, Medicare risk corridors allow a Part D plan sponsor that spends less than its bid estimate of costs to keep all savings up to 5% and a portion of those savings thereafter, which, in practice, allows PBMs and Part D plan sponsors to retain the vast majority of DIR fees collected. Thus, PBMs and Part D plan sponsors financially benefit from DIR fees.
Worse yet, DIR fees on expensive specialty drugs are typically calculated as a percentage of a drug’s list price. As such, DIR fees provide another incentive for PBMs to keep drug list prices high – high list prices yield not only larger rebates, but also larger DIR fees. As such, over the past several years DIR fees have become a larger percentage of the overall revenue that PBMs and Part D plan sponsors receive. Simply put, PBMs are making their money one way or another — rebates or DIR fees from pharmacy providers.
More problematic than the growth of DIR fees is the manner in which DIR fees are assessed on providers, especially community oncology practices. These fees are charged against community oncology practices based on their performance in a number of primary-care focused “quality metric” categories, which are totally unrelated and irrelevant to the cancer patients these practices treat. As a result, these community oncology practices have no meaningful ability to influence their performance scores – with no ability for upside – and such fees amount to nothing more than extortion from practices. Given the market clout of the top PBMs in terms of the percentage of prescription drugs they manage, community oncology practices simply have to pay these DIR fees to stay in network, lest they lose the ability to provide dispensing services to their patients.
These DIR fees are assessed after the point-of-sale. While they are sometimes recouped as soon as PBMs reimburse providers (i.e., extracted from initial reimbursements), in most cases DIR fees are assessed months after patients receive their medications. The total amount of DIR fees assessed on providers may not be known by providers until more than a year after a drug has been dispensed, as some PBM contracts create the potential for a partial or total refund of DIR fees (though a total refund is practically unobtainable).
DIR fees increase patients’ cost sharing responsibilities because patient out-of-pocket costs are based on an artificially inflated list drug prices at the point-of-sale; thus, in the case of Medicare patients, prematurely pushing them into the Medicare Part D “donut hole.” The cost of DIR fees also shifts the burden of drug costs to the federal government as more patients are prematurely pushed into the catastrophic phase of the Medicare benefit, resulting in higher financial contribution by the Medicare program. Ultimately, DIR fees weakens the overall benefit of the Medicare insurance benefit intended to provide health care coverage for our nation’s oldest and most vulnerable citizens.
Finally, DIR fees extracted from reimbursement to providers often results in drugs reimbursed below drug acquisition cost. Some speculate that this is yet another strategy by PBMs to ultimately drive pharmacy providers out of business so that the PBMs can take over the business with their retail, specialty, or mail-order pharmacies.
PBMs are able to effectively “extort” DIR fees due to their size and hegemony. As of 2018, three companies – UnitedHealth, Humana and CVS Health – covered over half of all Medicare Part D patients. Pharmacy providers do not have a meaningful choice but to accept the terms being provided to them – rejecting just one Part D plan could mean losing out on being able to service nearly a quarter of their Medicare Part D patients. PBMs know the power they hold and use it to its fullest extent.
5.1 Who is Impacted?
The expansion of DIR fees has had a substantial negative impact on both Medicare beneficiaries and the program as a whole. As confirmed in recent CMS studies, DIR fees ultimately shift financial liability from the Part D plan sponsor to the patient, then ultimately to the federal government, through Medicare’s catastrophic coverage phase. The shifting of financial liability away from the Part D plan sponsor and to Medicare and the patient is even more pronounced with specialty medications, such as oral cancer medications.
5.1.1 Harm to Patients
The primary harm to patients from DIR fees is that patients’ out-of-pocket costs are higher because they are based on list drug prices. Once again, PBMs have a vested financial interest to have drug list prices as high as possible as DIR fees are assessed as a percentage of the list prices for expensive specialty drugs. Medicare Part D patients find themselves paying more for their medications because they pay increased copayments and coinsurance on inflated point-of-sale list prices, which do not reflect the after-the-fact price adjustment in DIR fees that the PBM is clawing back from the pharmacy provider.
The use of DIR fees by PBMs has degraded the quality of the Medicare Part D benefit available for beneficiaries, all the while providing an additional lucrative revenue source for PBMs and affiliated Part D plan sponsors. It has shifted the benefit of the Medicare Part D program from those who rely on it for drugs, to those that do not use it, in the form of lower (or zero dollar) premiums. Meanwhile, DIR has put upward pressure on drug expenditures for those that use the benefit. Studies conducted by CMS have concluded that DIR fees increase out-of-pocket costs for Medicare patients at the point of sale.
Consider for example, that Medicare Part D beneficiaries’ cost sharing is based on the PBM-determined rate at the point-of-sale. DIR fees are by definition not assessed at the point of sale. Thus, the patient’s copayment or coinsurance that is based on the price at the point-of-sale is artificially inflated. CMS similarly concluded that DIR fees cost patients money, noting “[w]hen pharmacy price concessions and other price concessions are not reflected in the negotiated price at the point of sale (that is, are applied instead as [Direct and Indirect Remuneration] at the end of the coverage year), beneficiary cost-sharing increases.”
Likewise, up until the end of the 2020 plan year when the “donut hole” existed in the Medicare Part D Program, DIR fee programs pushed patients through the coverage stages much faster. Within the donut hole, patients pay 25% of the drug cost based on the (inflated) list price at the point-of-sale. The concern that patients continue to foot the bill for increased costs is not hidden from scrutiny as a group of 21 U.S. Senators urged HHS to address DIR fees because “beneficiaries face high-cost sharing for drugs and are accelerated into the coverage gap (or “donut hole”) phase of their benefit.”
In addition, despite PBMs’ purported justifications for such programs, DIR fees have not benefitted the quality of Part D plans offered to Medicare beneficiaries. For example, SilverScript had a 4.0 Star Rating from Medicare in 2018 (based on 2017 data), but saw its score drop to a 3.5 Star Rating in 2019 despite the widespread usage of DIR fees. At the same time, as the impact of DIR fees has increased dramatically since 2016, patients have also been impacted by diminished access to care as providers facing decreased net reimbursement are forced out of business, forcing patients to receive services from pharmacies owned by or affiliated with the very PBMs and Part D plan sponsors extracting DIR fees (see, Section 6, infra).
5.1.2 Harm to Plan Sponsors
Just as DIR fees negatively impact patients, PBM-Imposed DIR fees shift costs away from Part D plan sponsors, while increasing the costs to the Medicare program (and in turn, the taxpayer) for catastrophic coverage and subsidy payments. As mentioned, when a Medicare beneficiary is pushed through the benefits tiers and reaches the “catastrophic coverage” stage, the cost of services shifts to 80% paid by Medicare, while only 15% paid by the plan sponsors. The government covers these costs in part by turning to the reinsurance marketplace. From 2007 through 2018, a period similar to when CMS saw DIR fees from pharmacy price concessions increase by more than 45,000 percent, reinsurance costs of Medicare soared by 411%. Part D plan sponsors and their PBMs have a financial incentive to move Medicare beneficiaries into the catastrophic phase of coverage, to the detriment of the taxpayer.
In fact, the National Community Pharmacists Association (NCPA) commissioned a report by Wakely Consulting Group, LLC to estimate the cost savings that would occur if congress prohibited retroactive reductions in payments by Part D plan sponsors in the form of DIR fees. Wakely Consulting Group, LLC found $3.4 billion in Part D payments over a nine-year period if these fees were prohibited.
Unfortunately, the harm from DIR fees goes beyond the Medicare program and American taxpayers. Like rebates, DIR fees have the effect of driving up the cost of drugs, through higher list prices. From 2013 to 2019, DIR fees rose from $229 million to an estimated $9.1 billion. Most striking, however, is that DIR fees now account for more than 18% of all Medicare rebates received by Part D plans. This increased reliance on DIR fees relative to drug rebates, both of which are tied to the list price of drugs, highlights the upward pressure DIR fees have placed on list prices for drugs. During this same period, drug list prices grew between 10-15% per year. Meanwhile, net prices have been relatively flat throughout this time period. These inflated list prices are felt by all plan sponsors – especially employers and state Medicaid programs – who do not receive any of the supposed benefits of DIR fees (such as lowered premiums).
PBMs have used their consolidation in the marketplace to use DIR fees and rebates in concert, fueling higher drug prices, while adversely impacting cancer care.
5.1.3 Harm to Providers
To say that DIR fees have had an adverse impact on providers is an understatement. DIR fees decrease pricing transparency creating uncertainty as to the true real reimbursement rates for drugs, very often driving reimbursement rates below the providers’ acquisition cost of drugs (see, Section 8, infra).
The metrics utilized by PBMs in implementing DIR fee programs are typically completely inapplicable to community oncology practices. Specifically, community oncology practices dispense primarily (and almost exclusively) specialty medications for cancer patients. As such, they have virtually no ability to influence their performance based on PBMs’ “quality metric” categories measuring patient drug adherence relating to cholesterol, heart disease, and diabetes medications, which are relevant to dispensing general medications, not specialty drugs.
Worse yet, adherence-based metrics are particularly problematic and in cases not only wholly inapplicable in treating cancer patients, but also may be very dangerous. Community oncologists are extremely vigilant about monitoring their patients’ cancer medication regimens and may temporarily discontinue or “hold” medications until a patient’s status returns to an acceptable level, especially relating to adverse drug side effects. The period during which the medication is “held,” or therapy is temporarily discontinued, is wrongly and obtusely measured by the PBM as a lack of adherence in one of the few areas where the community oncology practices may be measured, ultimately causing the community oncology practices’ performance to decrease, and the DIR fee assessment to subsequently increase.
Consider, for example, Imbruvica (ibrutinib), which is dispensed by many community oncology practices to treat mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL). Studies have shown that Imbruvica tends to cause hematologic effects such as neutropenia and thrombocytopenia in MCL and CLL. If these adverse events occur at certain levels, the standard of care – as articulated directly by the FDA-approved package insert – is to hold the medication until the patient’s lab values return to normal ranges. This can happen in as many as 46% of cases, resulting in discontinuing the patient’s medication for up to a month. If community oncology practices are required to continue to dispense this drug, it will result in additional (and avoidable) costs to Medicare for the discontinued fills, as well as potential harm to the patient (along with potentially increased costs to Medicare for associated medical costs).
Further, due to the high cost of specialty drugs, and in particular, oncology medications, any small change in perceived adherence rates due to the purposeful physician-directed temporary discontinuation of therapy results in unreasonably low reimbursement rates. Many PBMs justify their DIR fee programs as being designed to influence providers to deliver better care to patients in their Medicare Part D networks. On that clinical basis, if community oncology practices were to be “influenced” by the PBMs’ DIR fee metrics by adhering to a medication when the FDA-approved label calls for the therapy to be held, patients would suffer. As such, community oncology practices are often left without any meaningful way to impact PBMs’ so-called “quality metrics” and improve their DIR fee performance.
Ultimately, community oncology practices have no way out. For them, due to the clout and market leverage of PBMs, DIR fees are simply a form of extortion that community oncology practices are forced to pay.
5.2 What Does the Law Say?
The most directly applicable legal principles relating to pharmacy DIR fees are found in the federal Any Willing Provider law. Within the federal Any Willing Provider law, CMS expressly recognized that unreasonably low reimbursement, which often result after accounting for DIR fees, violates the federal Any Willing Provider law. As it relates to the methodologies being used to assess DIR fees, performance criteria, and the manner in which PBMs and Part D plan sponsors are using those programs must also be reasonable and relevant. For community oncology practices, performance criteria that they are unable to influence or performance criteria that does not reasonably measure optimal cancer care can run afoul of the federal Any Willing Provider law.
In addition to explicit statutory language and CMS guidance, many of these principles are incorporated within, and apply directly to, the contract between PBMs and community oncology practices. PBM contracts include explicit obligations that the PBMs will comply with federal code, statues, rules, and CMS guidance, including but not limited to the Medicare Part D Provider Manual. These contractual obligations are not included in the contract with pharmacies by choice, but rather federal law requires these terms to be included in the contract between CMS and plan sponsors, and in contracts with their first tier entities (including PBMs, and in contracts between PBMs and pharmacy providers). This creates affirmative obligations on PBMs to comply with these laws, as well as the ability for pharmacy providers to directly challenge PBMs for breaches of contract when PBM actions do not comply with federal law.
In January 2022, CMS introduced a proposed Final Rule that would alter the way PBMs and Part D plan sponsors are required to report DIR fees. In particular, CMS has proposed that PBMs and Part D plan sponsors report the lowest possible reimbursement to pharmacy providers (inclusive of all potential DIR fees) as the “negotiated price.” While this proposed rule (if finalized) could have the result of removing the financial incentive for PBMs and Part D plan sponsors to institute retrospective DIR fees, it does little to protect pharmacy providers against unreasonably low reimbursement rates or wholly irrelevant “quality” metrics when assessing DIR fees.
5.3 What Can Be Done?
- Legislative Solutions
-
- Federal legislation should be enacted requiring that any DIR fee program (i) be tied to relevant quality programs to the specialty being measured; (ii) actually measured on an individual pharmacy level; (iii) provide equal opportunity for upside performance (i.e., not just a way for PBMs to “rig” the program to always measure downside performance resulting in DIR fees extracted from the provider); and (iv) require that DIR fees be applied equally and fairly across all network pharmacies, specifically including PBM-owned or affiliated pharmacies).
- Federal legislation should require that all pharmacy price concessions, including DIR fees, be included in the negotiated price at point-of-sale.
- Federal legislation should give CMS greater latitude in regulating the reimbursement structure between Part D plan sponsors and pharmacy providers.
- Regulatory
-
- CMS should issue regulation providing “guard rails” on what constitutes reasonable and relevant terms and conditions, and clarify that whether given terms are “reasonable” or “relevant” can be adjudicated in a private contractual dispute between Part D plan sponsors/PBMs and pharmacies.
- CMS should initiate complaints against Part D plan sponsors and PBMs who have failed to pass on negotiated prices to patients at the point-of-sale, when DIR fees were known or knowable (i.e., the PBM maintained a minimum range of DIR fees that were to be assessed against every pharmacy no matter what).
- CMS should initiate complaints against Part D plan sponsors and PBMs who have not paid providers based on reasonable and relevant terms and conditions, including through unreasonably low reimbursements, or irrelevant performance criteria.
- CMS should require reporting of pharmacy DIR fees by both NDC number and pharmacy National Provider Identifier (NPI) allowing for full end-to-end audits of the flow of money from pharmacies to the Medicare program. The results of these audits should be made available to the public.
Restrictive Networks, Credentialing Abuses, and Artificial Barriers of Entry
PBMs maintain a monopoly-like grasp on the industry, the natural result of which is the inability of patients to freely choose a provider based on his or her personal health care decisions, as opposed to the mandates of his or her PBM. As noted previously, only three PBMs process more than three-quarters of all prescription claims: CVS Health, Express Scripts, and OptumRx, while five PBMs process over 80% of all prescription claims. Each of the three major PBMs share common ownership with a major insurer and in turn with a mail-order and/or specialty pharmacy. These vertical, integrated relationships allow the PBMs to control the pharmaceutical supply chain, and erect superficial barriers to entry or even outright exclude entire classes of potential pharmacy providers.
This is particularly pronounced in the context of cancer care, where the introduction of new oncology therapies over the past several years, specifically, oral treatments for cancer and related conditions, presents new challenges for patients, plan sponsors, and providers alike. Between 2017 and 2019, there have been over twenty-four new oral cancer medications introduced into the marketplace. In 2020 alone, ten new oral oncolytics were approved by the FDA. As it stands, oral oncolytics make up 25% to 35% of cancer medications in development, making it likely that over the next several years, oral therapies will encompass an indispensable component of any treatment plan for cancer patients. While traditional chemotherapy infusion therapy that is “administered” is covered under a patient’s “medical” benefits, oral oncolytics that are “dispensed” are being shifted to the patient’s “pharmacy” benefits, managed by PBMs. Unlike chemotherapy administered in the clinic setting, the advent of oral oncolytics have given the PBMs a tremendous new opportunity to control cancer care and divert prescriptions and profits to themselves.
These new oral cancer medications can be extremely expensive, often ranging more than $10,000 per month. This is what is attracting PBMs, and as a result, PBMs have attempted to use their market size and leverage to limit dispensing of oral oncolytics through certain specialty and/or mail-order pharmacies, most often their own or affiliated pharmacy.
PBMs use several different tactics to maintain their control over where patients receive their care. The first and foremost of these is creating restricted networks, blocking access to any provider that is not affiliated with their PBM. In these instances, the PBM will contend that the network is “closed” or that there is no “network,” and thus, pharmacy providers are not even given the opportunity to apply for network admission. This occurs more frequently in the commercial insurance space involving employer-sponsored plans, but can also involve Medicaid managed care programs, where the PBM will require patients to receive their cancer medication from the PBM’s wholly-owned or affiliated pharmacy, and no one else. This is anticompetitive conduct – pure and simple – where patients are trapped into using one particular provider not based on the quality of care provided by that provider but based on the financial arrangements and the corporate affiliation between the pharmacy provider and the PBM and/or health insurer.
A related, but slight variation of this tactic is to restrict access to certain classes of providers (i.e., retail pharmacies), while excluding wholesale other classes of providers (i.e., dispensing physician practices). For example, beginning in early 2016, CVS Caremark espoused a self-serving stance that dispensing physician practices were now to be deemed “out-of-network” and no longer able to participate in Medicare Part D networks. This would have the effect of dramatically interrupting the ongoing relationship between treating oncologists and their patients. CVS Caremark later backtracked on this position and began allowing “grandfathered” dispensing physicians (i.e., those that previously held a contract with the PBM) to continue in-network, but delayed the processing of any new, non-grandfathered dispensing physician practices. In another instance, in January of 2018, Prime Therapeutics (Prime) – the PBM owned by a consortium of approximately twenty-two Blue Cross Blue Shield plans – announced that it would no longer accept any new dispensing physicians into its pharmacy networks on the alleged basis of “fraud, waste, and abuse” concerns and a commitment to maintaining to compliant networks. Without providing any further details, Prime claimed that Dispensing Physicians did not adhere to Prime’s Provider Manual. This trend expanded to existing in-network dispensing physicians actively servicing patients when, recently, Prime announced that it would also terminate existing, or “grandfathered” dispensing physicians from its networks. Despite having credentialed, contracted, and paid dispensing physicians as “in-network” Medicare Part D providers for over a decade, Prime seemingly unilaterally took the position that dispensing physicians are now considered “out-of-network providers” under Medicare Part D. Like wholesale network exclusion, these practices disadvantage vital providers while allowing PBM-owned or affiliated pharmacies to capture a greater share of prescription volume.
Even in instances where a PBM nominally allows a community oncology practice to apply for network participation, the PBM can still place other barriers in the way of providers being able to service their patients by imposing onerous credentialing processes. For a community oncology practice to service patients within a PBM’s network, PBMs require that the provider adhere to specific and extremely onerous, credentialing requirements, including the requirement that the provider maintain certain accreditations. These conditions are made even more onerous where PBMs delay the review of credentialing applications (seemingly with the intention to avoid admitting these providers), enact credentialing applications with terms and conditions designed to keep out providers (rather than ensuring the quality of providers) or allow participation but at rates so low that reimbursement may not even cover the acquisition cost of a drug. These obstructionist policies harm patients, degrade the quality of prescribers and benefit only PBMs that are incentivized to continue to these illegitimate practices.
Finally, even when a community oncology practice has ultimately been admitted into a PBM’s network, PBMs continue to utilize other tactics to drive patients away from community oncology practices, and towards PBM-owned or affiliated pharmacies. This includes tactics such as patient slamming and claim hijacking (see, Section 7, infra), misleading communications aimed at steering patients to PBM-owned or affiliated pharmacies, and creating patient incentives for patients (such as lower copays, larger days’ supply or free products/services) to utilize preferred PBM-owned or affiliated pharmacies. PBMs also utilize other tactics, such as abusive auditing practices (i.e., requiring the production of thousands of pages of documentation to support claims billed) and terminating providers without cause or on pretextual bases (i.e., that they only dispense one class of medications).
PBMs employ these tactics to maintain their oppressive market dominance. But at the same time, in a vicious cycle, these tactics are themselves the consequence of the horizontal and vertical consolidation within and between insurance and PBM markets, which has created merged entities with such oppressive power that it a virtual chokehold on community oncology practices and pharmacy providers. The result of these tactics is that patients are steered away from receiving care at their community oncology practices, and forced to receive care from PBM-owned or affiliated pharmacies. This is not only without regard to the impact on patient care and outcomes, but as the chart below demonstrates, only continues to prop up higher drug prices and charges.
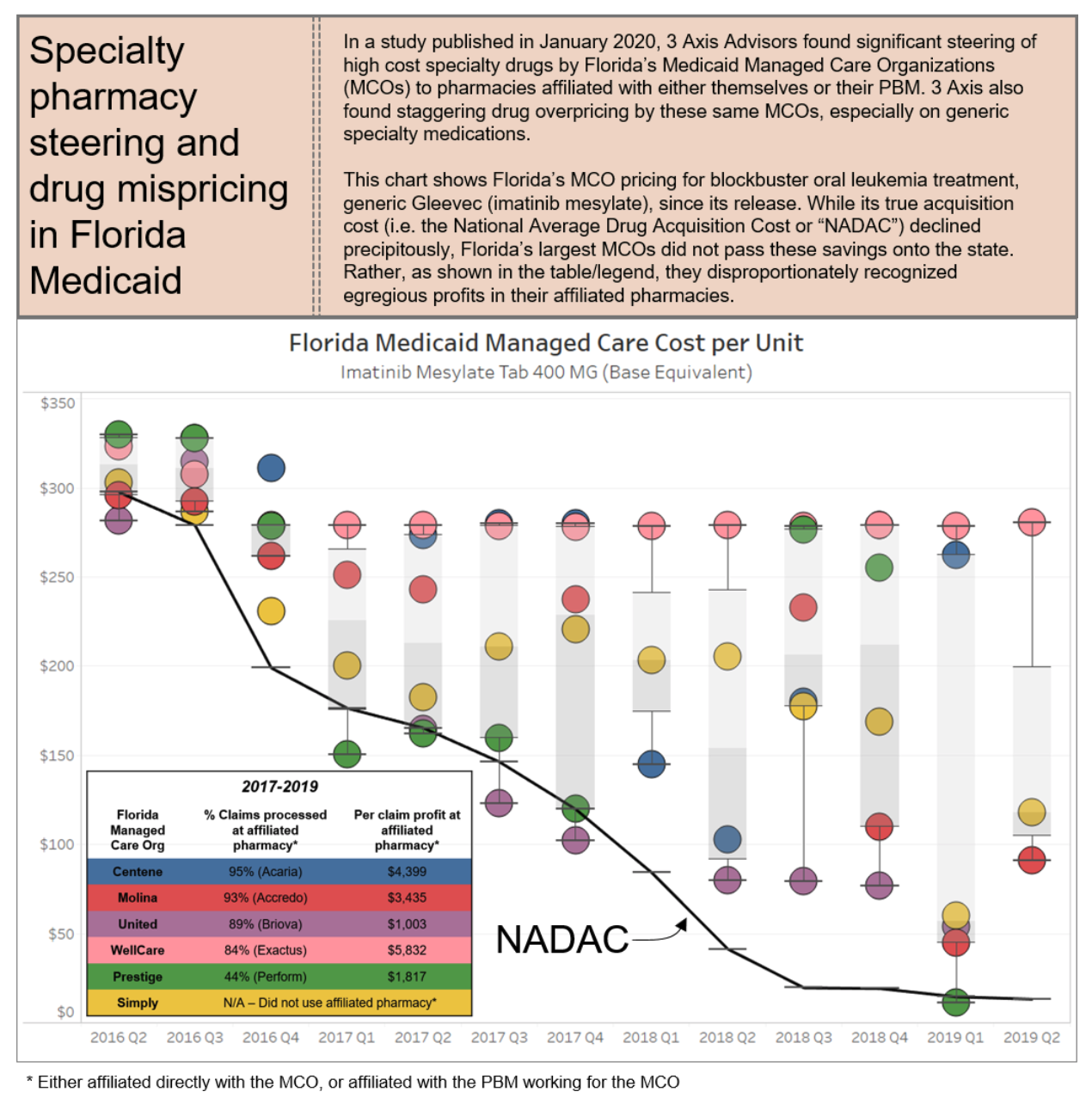
6.1 Who is Impacted?
The overall lack of industry standards and oversight in the PBM credentialing sphere has led to arbitrary denials and lengthy, costly application processes, that ultimately have a negative impact on a community oncology practice’s ability to focus on patient care. Instead of allowing community oncology practices to enter into their networks, PBMs attempt to limit the dispensing of oral oncolytics through their own specialty pharmacies, leading to poor patient compliance and adherence to life-saving treatments, causing the quality of cancer care to suffer.
These tactics have had negative impact all across the spectrum, affecting patients, health care payers (including Medicare, Medicaid, employers and taxpayers), and providers.
6.1.1 Harm to Patients
These exclusionary practices – whether they be unreasonable barriers to entry or outright exclusion of certain classes of providers – result in serious harm to patients, specifically those who are seeking the services of community oncology practices that have been excluded from a PBM specialty network. For one, these exclusionary practices destroy existing patient-provider relationships. In early 2016, when CVS Caremark undertook re-interpreting longstanding CMS regulations, it did so in such a way as to effectively cut out physicians from continuing to dispense medications to their existing Medicare Part D patients. PBMs have no regard for the continuity of these vital health care relationships and their impact on patients’ well-being and outcomes.
This is critical, as patients are more likely to raise certain questions or concerns about their medications, when these medications are dispensed by community oncology practices. To strip patients, who are facing serious life-threatening diseases, of that important patient-provider relationship could result in serious patient harm. This also has the effect of decreasing medication adherence, which would further affect patients, especially those undergoing life-saving treatments at community oncology practices.
The ultimate outcome of creating restricted networks or excluding entire classes of providers, namely, that patients are essentially required to obtain medications at a PBM-owned or affiliated pharmacy. It is well-documented that when the PBM-owned or affiliated pharmacy is responsible for filling the patients’ prescriptions, it results in worse care. The near-monopolistic control of the network, combined with the lack of patient choice, remove any checks and balances on the quality of the care being provided.
Consider, for example, a patient battling cancer was denied life-saving medications by a PBM due to the PBM being unwilling to enter medications into its computer system. In another example, a patient had been diagnosed with Philadelphia chromosome-positive + chronic myeloid leukemia and had been responding positively to “180mg” of a certain medication. However, according to the patient’s PBM, the medication had to come from the PBM’s mandated mail order specialty pharmacy instead of a pharmacy of their choice. Since the medication was not available in a single 180mg dosage form, the prescription clearly indicated that the patient was to receive a “100 mg tablet and an 80 mg tablet.” Instead, over the course of the next several months, the PBM pharmacy dispensed either a 100 mg tablet or an 80 mg tablet, but never both. Ultimately, the patient did not respond well to the lowered dosages of the medication. Finally, in a particularly disturbing example, a colorectal cancer patient was prescribed a common oral medication that had been on the market for nearly twenty years. The patient’s PBM mandated that the patient fill the prescription at a large, well-known specialty pharmacy, and the patient’s oncologist prescribed the medication to be taken in rounds with the following specific instructions: ‘two weeks on, one week off.’ The PBM mail-order pharmacy neglected to include the ‘one week off’ instruction on the label, and as a result, the patient ended up in the intensive care unit of a hospital.
Unfortunately, patients often do not have any ability or choice to switch their PBMs in order to have control over which pharmacy provider from whom they would like to receive service. PBMs who undertake these restrictive practices are typically selected by the patient’s employer (or sometimes by the insurance company selected by the patient’s employer). The patients are two, sometimes three steps removed from any part of the decision-making process. Since most patient get their health care coverage through their jobs, the only way a patient can exert any control over the network of pharmacy providers is to change jobs and hope that their new employer utilizes a different PBM’s network. But, in a world where three PBMs account for nearly 80% of the marketplace, the odds of getting a better PBM are slim to none.
The PBMs know the level of power that they wield. And their focus is on profits, not patients. Ultimately, given the acute focus on patient care inherent in community oncology practices, patients suffer when those providers are forced out of the space.
6.1.2 Harm to Plan Sponsors
In addition to patients, these exclusionary practices harm plan sponsors, such as Medicare and Medicaid, because they cause an artificial rise in the cost of specialty medication, particularly within the oncology space. Specifically, the exclusion of community oncology practices from PBM networks require more patients to utilize PBM-owned or affiliated mail-order and/or specialty pharmacies. This, in turn, leads to exponentially more waste of medication, causing increased costs to plan sponsors. Mail-order pharmacies, without proper access to patient outcomes, routinely dispense 90-day supplies of medications. In several instances, patients continue to receive medications despite their repeated requests to have the mail-order pharmacy cease sending medication, often due to a change in their course of treatment. In more tragic cases, the PBM mail-order pharmacies continue to dispense medications to the patient’s residence despite the patient having passed away, leading to the waste of unwanted, expensive medications.
Moreover, when pharmacy care is diverted from community oncology practices to PBM-owned or affiliated pharmacies, plan sponsors lose out on tremendous value-based contracting opportunities. In the Medicare space, CMS is developing new payment and delivery models designed to improve the effectiveness and efficiency of specialty care. Among those specialty models is the Oncology Care Model, which aims to provide higher quality, more highly coordinated oncology care at the same or lower cost to Medicare. The Oncology Care Model “provides an incentive to participating physician practices to comprehensively and appropriately address the complex care needs of the beneficiary population receiving chemotherapy treatment and heighten the focus on furnishing services that specifically improve the patient experience or health outcomes.” PBM exclusionary practices would thwart this initiative. Likewise, in the private sector, value-based care (VBC) innovations are on the rise, increasing the quality while lowing the overall cost to health care payer and their patients. The ability to tie benefits to providers and value to patients is critical to aligning interests in the health care space and has long been a long-term goal of health policy experts. However, this type of integration of medical and pharmacy care is against the interest of current PBM practices to implement. Absent changes to PBM regulation, the federal government will be unable to achieve some of the same cost-saving/quality improving measures as is being utilized in primarily the self-funded employer sponsor health care space.
Unfortunately, these lost opportunities are not made up for in savings garnered by PBMs, and in fact, quite the opposite has occurred. As illustrated in the figure on page 36, the exclusion of community oncology practices and other independent providers allows PBMs to pocket more through their wholly-owned or affiliated mail-order and specialty pharmacies.
In a study conducted by Ohio’s Medicaid Managed Care Pharmacy Services, PBMs billed taxpayers 8.8% more for medications than what they paid pharmacies. This difference, commonly referred to as “spread” has been growing and is typically the highest on specialty medications, such as oral oncolytics. Worse yet, similar data has shown that the spread between plan sponsor funded PBM revenue and pharmacy-captured reimbursement has increased over time. In short, PBMs are keeping more and more revenue from health care costs to the detriment of others in the health care space.
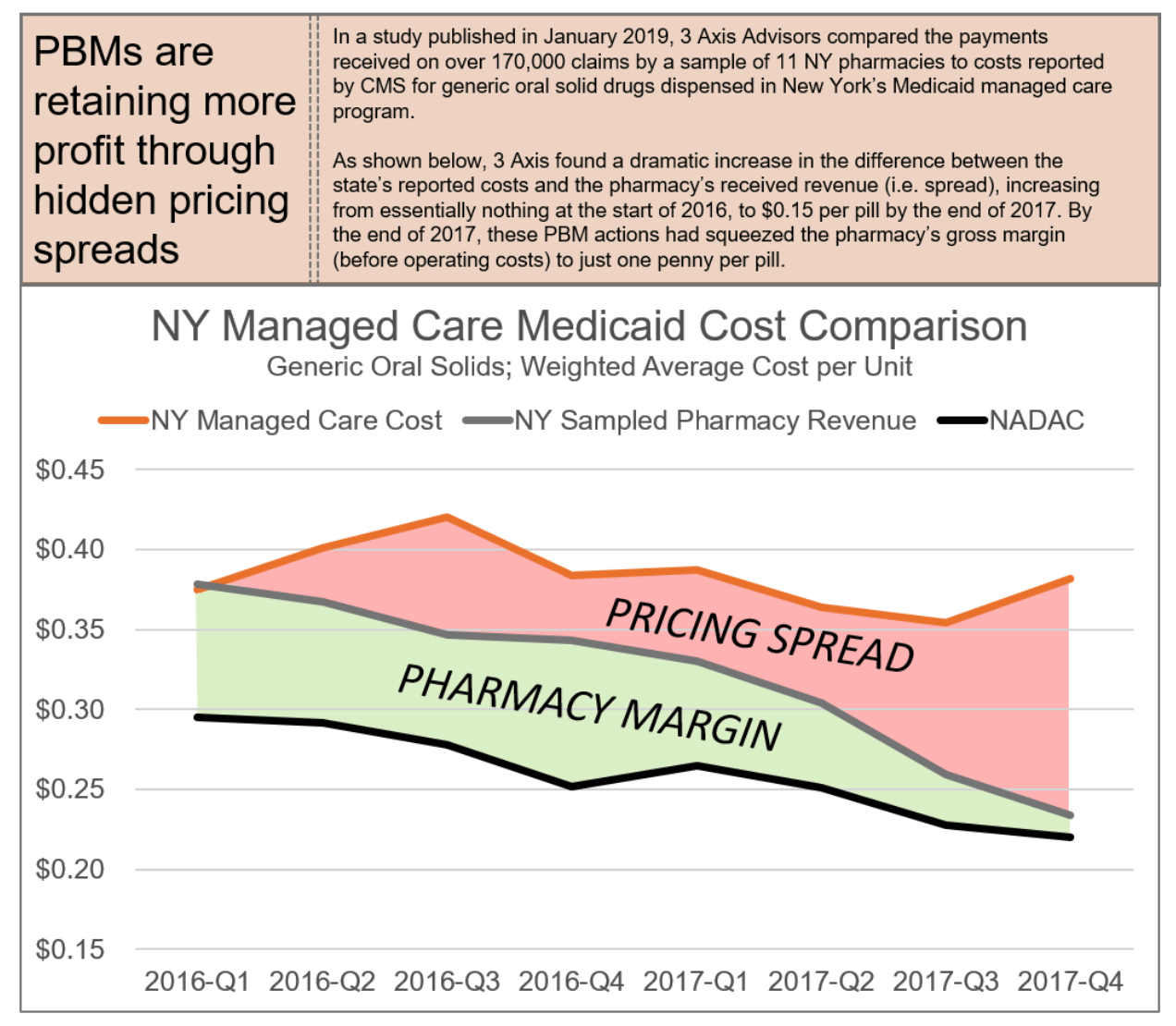
Ultimately, when compared to costs of PBM exclusionary practices, the savings associated with dispensing by community oncology practices are palpable. Reports estimate that physician point-of-care dispensing could save seniors and taxpayers over $20 billion in Medicare Part D alone.
6.1.3 Harm to Providers
An increasingly important component of the physician-patient relationship with oncology is the dispensing of medications to patients through the community oncology practice, at the site of care. Excluding community oncology practices from PBM networks prevents physicians from providing consistent care to their patients.
When PBMs impose unreasonably high or arbitrary requirements for network admission, designed for no purpose other than to serve as an artificial barrier of entry, they place immense and undue burdens on community oncology practices seeking to service their patients. As noted above, these credentialing standards often require a provider to hold multiple forms of accreditation, such as URAC and ACHC. These specified accreditations are often not the most relevant or appropriate form of accreditation for community oncology practices, and do not constitute the most applicable form of endorsement based on the unique and specialized services provided by community oncology practices.
Between the standards set forth under the Oncology Care Model (OCM) and Quality Oncology Practice Initiative (QOPI®) Certification Program, community oncology practices also attain high standards of practices, validated by third parties, that obviate the need for separate accreditation. For example, QOPI has a certification program specifically designed for clinical oncology practices as this process “can routinely evaluate practice performance against quality measures and standards established by experts in the oncology field.” Likewise, through the CMS-created OCM, community oncology practices have entered into payment arrangements that include financial and performance accountability for episodes of care surrounding chemotherapy administration to cancer patients. The practices participating in OCM have committed to providing enhanced services to Medicare beneficiaries such as care coordination, navigation, and national treatment guidelines for care. The fact that CMS has involved itself in the creation of this type of model with standards that directly correlate to community oncology providers demonstrates that these two programs (OCM and QOPI) would be the best industry standards to judge a network provider. Moreover, requiring dual accreditation – including URAC accreditation in Specialty Pharmacy – apart from being redundant, also increases the risks that the provider will have multiple, sometimes contradictory compliance requirements, needing to comply with not just ACHC standards, but also URAC standards, which at times can be diverging. Finally, these accreditations can be prohibitively expensive and costly, making it impracticable for providers to undertake the steps necessary to even seek admission to the networks.
Likewise, when PBMs take steps to delay credentialing, this too harms pharmacy providers. Community oncology practices have to divert considerable amount of time and resources to respond to repeated follow ups on their credentialing applications under normal circumstances. However, when a PBM “slow rolls” an application and takes months to review and respond to inquiries, this has often led to the PBM asking the provider to provide the same documentation over, and over and over again (i.e., licenses that expire and are renewed over the course of the sometimes 18-month long credentialing process). This takes time away from being able to service patients.
But perhaps the most direct way providers are harmed by these tactics is through the actual effects of network exclusion. Due to the size and market share of each PBM (see, Section 3, supra), a PBM termination or exclusion often spells irreparable harm for a provider seeking to participate in pharmacy networks and/or the Medicare Part D program. Particularly alarming is the fact that about two-thirds of all Medicare Part D Prescription Drug Plan enrollees are concentrated in networks across just three payers: OptumRx, CVS Caremark, and Humana. Exclusion from any one of these payers could make dispensing simply not a viable option for a community oncology practice.
6.2 What Does the Law Say?
Among all the barriers that PBMs put in front of providers – including onerous credentialing processes, restricting network access, steering to owned or affiliated pharmacies – the core legal principles largely tie back to rules promulgated around freedom of patient choice and network participation. Remarkably, there are several federal and state laws on the books that seek to safeguard the rights of patients to select the provider of their choice, or to protect community oncology practices from undue network termination or exclusion. In the federal statutes establishing and governing the Medicare program, Congress has included explicit “Any Willing Provider” requirements, which relate directly to network access for Medicare providers, including community oncology practices. These statutes apply to all Part D plan sponsors, as Part D plan sponsors are under the purview of CMS, pursuant to contracts between the Part D plan sponsors and CMS.
The Medicare Any Willing Provider law (42 U.S.C. § 1395w-104) explicitly requires that all Part D prescription drug plans permit “the participation of any pharmacy that meets the terms and conditions under the plan.” The federal “Any Willing Provider” law further prohibits health insurers from creating exclusive provider networks – or unduly barring entry to such networks (such as through artificial barriers of entry) – to which insured patients are directed to the exclusion and detriment of non-network providers. In fact, as it relates to credentialing abuses, CMS has also questioned whether mandatory accreditations should be considered “standard terms and conditions” of a network, and whether PBMs should instead explore other reasonable and relevant alternatives to ensure quality assurance and actual improved patient care, particularly where certain accreditation requires may be arbitrary and not directly proven to ensure quality assurance.
Likewise, federal law provides protection directly for patients to have the freedom to select a provider of their choice. Pursuant to 42 C.F.R. § 431.51(a), Medicaid beneficiaries may obtain services from any qualified Medicaid provider that undertakes to provide services to them. However, plan sponsors commonly use preferred networks to incentivize beneficiaries to fill claims at pharmacies of the Plan’s choice (rather than the beneficiary’s choice), by offering reduced co-pays at preferred pharmacies.
Several states also maintain their own versions of “Any Willing Provider” protections. For example, North Carolina’s Any Willing Provider Law provides that a health benefit plan shall not “[p]rohibit or limit a resident of th[e] State … from selecting a pharmacy of his or her choice when the pharmacy has agreed to participate in the health benefit plan according to the terms offered by the insurer,” or “[d]eny a pharmacy the opportunity to participate as a contract provider under a health benefit plan if the pharmacy agrees to provide pharmacy services that meet the terms and requirements, including terms of reimbursement, of the insurer under a health benefit plan…”
Similarly, Tennessee’s Any Willing Provider Law provides similar limitations on the ability to exclude providers such as community oncology practices, mandating that “[n]o health insurance insurer and no managed health insurance insurer may… deny any licensed pharmacy or licensed pharmacist the part to participate as a participating provider in any policy, contract, or plan on the same terms and conditions are offered to any other provider of pharmacy services under the policy, contract or plan” or “[p]revent any person who is a party to or a beneficiary of any policy, contract, or plan from selecting a licensed pharmacy of the person’s choice … provided that the pharmacy is a participating provider under the same terms and conditions of the contract, policy or plan as those offered any other provider of pharmacy services.”
These laws prohibit not just outright network exclusion, but also a host of other PBM practices aimed at requiring that patient use their wholly-owned or affiliated pharmacies.
At both the federal and state levels, policy recognizes the importance of provider access and, ultimately, competition via the enactment of these “Any Willing Provider” rules. Unfortunately, these laws have not been without attack by the powerful PBMs, and in few instances do they provide pharmacies a private right of action to enforce and ensure they are meaningfully applied.
6.3 What Can Be Done?
- Legislative
-
- Congress should enact federal legislation that provides a private right of action for community oncology practices to exercise their rights under the federal Any Willing Provider law, particularly when they are unfairly excluded from PBM networks and a private right of action will allow the enforcement of a regulation by a private party, such as a community oncology practice, allowing for litigation or the threat of litigation to incentivize compliance of the law.
- Congress should enact state legislation that curbs credentialing abuses and provides for stronger Any Willing Provider laws and provides for a private right of action for community oncology practices to exercise.
- Regulatory
-
- CMS should pursue complaints against PBMs for their construct of artificial barriers of entry and failure to adhere to the establishment of reasonable and relevant terms and conditions of participation.
- CMS should also enact regulation to specify “reasonable” and “relevant” standards of participation to allow for defined requirements PBMs must adhere to.
- CMS should issue regulation providing “guard rails” on what constitutes reasonable and relevant terms and conditions, and clarify that whether given terms are “reasonable” or “relevant” can be adjudicated in a private contractual dispute between Part D plan sponsors/PBMs and pharmacies.
- State Departments of Insurance should pursue complaints against PBMs for violations of Any Willing Provider Laws, and Medicaid Free-Choice-of-Provider provisions.
- Plan Sponsor Action
-
- Plan sponsors should require PBMs to seek approval from plan sponsors prior to establishing a standard and/or qualification for a provider network.
- Plan sponsor should have the full and final authority to make any modification to a standard and/or qualification for a provider network.
- Plan sponsors should retain the right to participate in an administrative hearing requested by a provider who has been terminated or rejected from a PBM’s provider network.
- Plan sponsors should retain the full and final authority to make accept or deny a provider’s request to participate in a PBM’s provider network.
Prescription Trolling, Patient Slamming, and Claim Hijacking
A patient’s decision on where to fill his or her medication, especially a cancer medication, is of immense importance. Cancer patients require ease of treatment and as little confusion as possible, in order to have a positive outcome. Based on these principles, Section 30.2.2.3 of the Medicare Prescription Drug Benefit Manual prohibits PBMs and Part D plan sponsors from “Steering of physicians or beneficiaries to a sponsor’s and/or PBM’s own mail order Pharmacy.” Such prohibition specifically includes steering of prescribers’ patients to a specialty pharmacy owned by or affiliated with a plan sponsor/PBM and most PBM contracts require adherence to CMS Guidance and contain compliance with law provisions.
Despite the law, there are innumerable instances where the PBMs haves effectively utilized claims or fill data and sought to move the prescription away from the provider of the patient’s choice and toward the PBM’s wholly-owned or affiliated pharmacy. This practice, sometimes referred to as “prescription trolling,” “patient slamming,” or “claim hijacking,” plays out fairly consistently. A typical case might involve a situation where the PBM allows the provider to submit a claim (typically a high-cost specialty medication), then reject it claiming that it required a prior authorization (PA). Then, once the provider has done all the required work to obtain the approval for the PA, it is subsequently rejected once again by the PBM, this time for the apparent reason that it “must” be filled at the PBM-owned or affiliated specialty pharmacy.
Pharmacy providers typically transmit prescription claims (and sometimes PA requests) to the patients’ PBM for purposes of having it adjudicated and receiving reimbursement. Such transmissions clearly contain protected health information (PHI) and are directed solely at the PBM acting as the claims adjudicator. Instead of simply reviewing and processing this claim, in its fiduciary capacity as the PBM, the PBM improperly and unlawfully accesses the PHI, and illegally communicates the claim information to its related entity (a PBM-owned specialty pharmacy). While the PBM is processing the PA, the PBM-owned or affiliated pharmacy surreptitiously communicates to the patient, prescriber, or both, with the goal of having the prescription filled at the PBM-owned or affiliated specialty pharmacy. Community oncology practices have documented some egregious instances where the PBM blatantly lied to the patient and pharmacy staff, saying the prescribing physician had authorized the transfer, when in fact, they clearly had not. Further, with complete disregard to not only patient privacy laws, but also state Pharmacy Practice Acts, PBM-owned specialty pharmacies have brazenly filled and dispensed the medication in complete absence of having an actual, signed prescription in hand.
Worrisomely, more deceitful and underhanded variations of this also exist. In some instances, PBM-owned or affiliated pharmacies have sought to mislead patients into thinking that their physician wants the prescription to be filled at the PBM-owned or affiliated pharmacy, or otherwise imbed prescription transfer documentation in the information the PBM provides to the physician in order to renew the prescription for refill (and the physician unknowingly signs to have the prescription transferred).
7.1 Who is Impacted?
7.1.1 Harm to Patients
A direct result of prescription trolling is severe confusion and distress for cancer patients, who are caught in the middle, uncertain of when or from where they will receive their next dose of their life saving medication. These concerns in the context of prescription trolling go beyond those when a PBM takes steps to create a restricted network (see, Section 6, supra); it is far more insidious here. While patients cannot be compelled to fill their prescription from a specific dispenser, many report receiving correspondence from their PBM implying that they must use a pharmacy owned by or affiliated with the PBM. These letters often explain that the insurance company has its own “preferred” pharmacy, from which the patient may already be receiving other prescribed drugs and offer for the patient to also get their oral cancer drug from this same source. PBMs may try to entice patients to select their “preferred” pharmacy through lower patient copayments to the patient only for the patient to later realize their oral oncolytics cost more at the “preferred” pharmacy than a non-preferred provider. Many patients find this confusing and do not understand the repercussions that jeopardize the monitoring, care control, and clinical management that they receive at their community oncology pharmacy, and they mistakenly, or unintentionally, switch their drug dispenser.
Many patients may require special assistance from their community oncology practice that has documented and understands their medical history, monitors for drug interactions between their medications, and is able to make appropriate dosing adjustments at the time of administration. Furthermore, a patient who is switched over to a PBM-owned or affiliated mail-order pharmacy often has his/her medication shipped from a distance (sometimes several states away), running the risk that the drug could be rendered ineffective in treating that patient’s condition due to a lack of sufficient temperature control during transit. In short, the harm can literally be deadly for patients with cancer, because of the disease and drugs involved – medications arriving too late or failure to timely amend dosing regimens can be the difference for life and death for these patients.
Perhaps worst of all, PBMs and their wholly-owned or affiliated specialty pharmacies have been known to employ underhanded tactics to “hijack” the prescription. In one particularly egregious instance, a PBM-affiliated specialty pharmacy contacted a community oncology practice claiming that one of the clinic’s patients had requested that his lung cancer medication be transferred to the PBM-affiliated pharmacy and demanded the clinic’s immediate compliance in the matter. Surprised by the news, the oncologist contacted the patient to inquire about his decision, only to discover that this was the first time the patient had heard of the matter. “Please do not transfer it anywhere else!” the patient requested. “I want to get it filled through the dispensary. I did not ask for this. I love being able to get this right away and with no hassles. I was on an oral chemo before and it was filled by a specialty pharmacy and I always was getting it late, missed a few days of medication sometimes and had numerous phone calls from them. They never seemed to know what was going on with my medication.” As evidenced by this true story account, patients receiving their oral drugs from a community oncology practice have access to those drugs within 24 hours of prescribing, and they can begin treatment immediately. Patients receiving their oral cancer drugs through a PBM, on the other hand, often have a much longer wait, sometimes 14 days or more. In addition to the delays, it is clear the oncology practices have access to patient records and can more closely monitor patients which empowers them to provide the most coordinated care.
In the end, the PBMs’ lack of transparency to the patient and the general public usurps the patient’s right of choice and circumvents the prescriber’s orders and independent professional judgment.
7.1.2 Harm to Plan Sponsors
The greatest harm to plan sponsors stemming from prescription trolling and claims hijacking is increased potential for waste, particularly compared to when the claim would otherwise be filled by the community oncology practice. Many times, a community oncology practice can identify certain medications that may be difficult to tolerate or patients whose conditions may require multiple dosing refinements. In these cases, in anticipation of such modifications, practices will often dispense a 15-day supply rather than a 30- or 90-day supply. PBM specialty mail order pharmacies can lack the expertise for such forethought or do not have the experience with care management to know when a smaller supply might be the wiser, more economical choice.
Ultimately, mandatory diversion of patients to PBM mail order pharmacies leads to increased waste of often-expensive and unwanted medication, thereby increasing overall health care spending, at the expense of Medicare and taxpayers. In a study funded by the Community Pharmacy Foundation reviewing medications being returned for disposal and destruction, it was found that prescriptions originating through mail order were far more likely to have excessive amounts of unused medication remaining (i.e., 80% or more of the prescribed quantity) when compared to retail pharmacies. In the cancer space, these issues of waste can be extremely costly. ln a particularly well-documented instance, a battling advanced colorectal cancer was told that his health plan would only cover his prescription for oral oncolytics if he obtained them through the PBM’s mail-order pharmacy. After he waited nearly two weeks to receive his prescription, when it finally came, it included incorrect dosing instructions, and he was told by the PBM-owned pharmacy to send back the medication (worth $20,000) so it could be destroyed. Even when the medication was ordered again, it came with fewer pills than were prescribed. While the PBM-owned or affiliated pharmacies continue to make errors and cause patients to endure life-threatening delays, the plan sponsors – like employers and Medicaid programs – are left footing the bill for these wasted products to the tune of tens of thousands of dollars in this one instance alone.
7.1.3 Harm to Providers
In addition to circumventing the prescriber’s orders and independent professional judgment, the PBMs’ tactics of prescription trolling further serves to push the burden of performing the initial administrative functions on to the community oncology practices, while removing any attendant benefits, as the first fill is the most expensive claim. The first fills of a prescription are typically a pharmacy’s most expensive claims due to several factors, including coordination with prescriber, prior authorization efforts, researching and liaising with patient assistance programs, engaging in patient training and providing skilled nursing administration. And further, at its core, through these claim rejections, the PBMs are once again depriving providers of any ongoing and expected future business relationships with patients who initially sought to fill prescriptions with their provider.
Apart from just the lost revenue, at their core, these tactics create a lot more work for already burdened community oncology practices and make patient treatment much more difficult. In the course of the PBMs’ efforts jockeying for control of the prescription, staff at community oncology practices spends hours on the phone with all the disconnected and disjointed stakeholders, just trying to get the prescription filled and in the patient’s hands. This includes speaking with the PBM, then the insurance company, then the PBM-owned or affiliated pharmacy, then the PBM again – and this all assumes everything goes “smoothly.” It is well-documented that these additional layers of unnecessary administrative complexity burden the health care system, with health care stakeholders spending about $496 billion on billing and insurance-related costs each year. These additional administrative burdens have been found to have a direct negative impact on patient care.
Yet PBMs remained focused on maximizing profits. As the chart below show, immense profit comes along with diverting prescriptions to PBM-owned pharmacies. Within the Florida Medicaid program, the overwhelming majority of “profits” earned from dispensing brand name drugs (including cancer medications) was retained by just three PBM-owned or affiliated pharmacies.
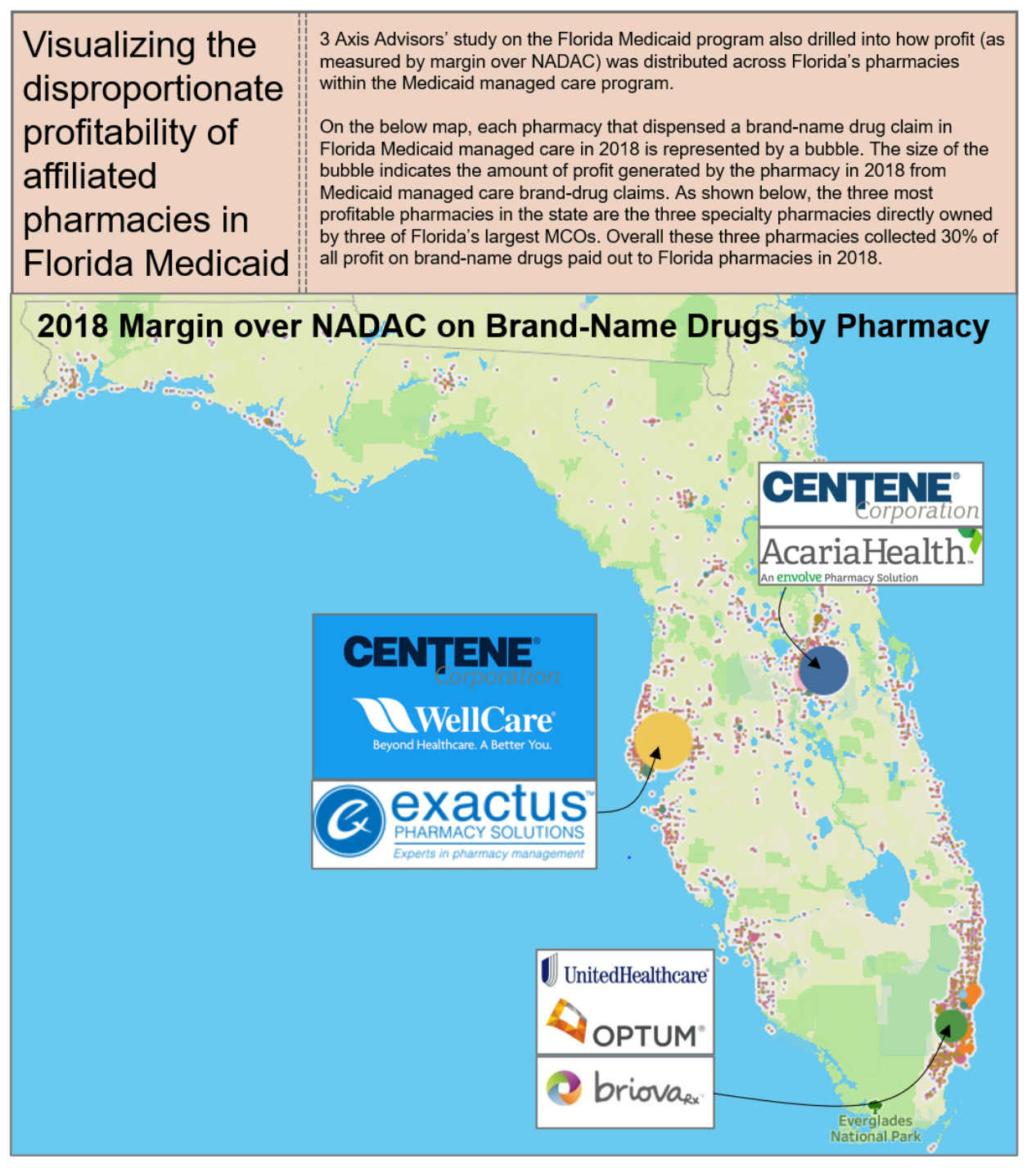
The combination of restricted networks, prescription trolling, and the mandating of dispensation of specialty drugs at specific pharmacies has been a boon to the specialty pharmacy arms of the nation’s largest insurers and PBMs, driving disproportionate profit to them vis-à-vis their unaffiliated pharmacy peers.
7.2 What Does the Law Say?
In addition to federal and state Any Willing Provider and Freedom of Patient Choice laws, which are certainly implicated by PBMs directing patients to their wholly-owned or affiliated pharmacies and excluding community oncology practices (see, Section 6, supra), several other federal and state laws bear on the tactic of prescription trolling. First and foremost, this activity runs afoul of the Health Insurance Portability and Accountability Act and the regulations promulgated thereunder (HIPAA), which limit the disclosure of PHI by covered entities, including pharmacies and PBMs, without patient authorization. In the absence of a valid authorization, disclosures of PHI may only be made for purposes of treatment, payment, or health care operations of the covered entity. As such, a PBM’s access to and use of PHI to steer patients toward the PBM’s wholly-owned or affiliated pharmacy is a breach of HIPAA, and compromises the privacy and security of patients’ personal information. HIPAA provides, in addition to substantial civil penalties, criminal sanctions for the use of PHI in this way, which demonstrates the significance of maintaining patient privacy.
In addition, these practices likely violate many states’ Anti-Patient Steering Laws which prohibit PBM or insurer-owned or affiliated pharmacies from “steering” profitable prescriptions to their own affiliated PBM and insurance pharmacies. For example, Louisiana provides that a PBM shall not directly or indirectly engage in patient steering to a pharmacy in which the PBM maintains an ownership interest or control without making a written disclosure and receiving acknowledgment from the patient; and the PBM is further prohibited from retaliation or further attempts to influence the patient, or treat the patient or the patient’s claim any differently if the patient chooses to use the alternate pharmacy. Likewise, New Jersey makes it unlawful for a pharmacist to enter into an arrangement with a health care practitioner who is licensed to issue prescriptions, or any institution, facility, or entity that provides health care services, for the purpose of directing or diverting patients to or from a specified pharmacy or restraining in any way a patient’s freedom of choice to select a pharmacy. When the PBM engages in these underhanded tactics, it is not only directly steering the patient to a particular pharmacy without their knowledge or consent, but forcing the community oncology practice to go along with the scheme, by consenting to transfer the prescription.
Lastly, even beyond state laws, prescription trolling may impinge on other federal requirements, including Section 2 of the Sherman Act (i.e., attempted monopolization using their role and leverage as PBM gatekeeper to divert business to the PBM-owned or affiliated pharmacy), and the Employee Retirement Income Security Act of 1974 (ERISA) and its requirements that fiduciaries discharge their duties with respect to the plan solely in the interest of the participants and beneficiaries (misappropriate PHI for pecuniary gain certainly could arise to the breach of a fiduciary duty for PBMs).
The overarching legal principles are potentially tempered somewhat by recent case law involving PBM appropriation of claims data. In Trone Health Servs., Inc. v. Express Scripts Holding Co., No. 4:18-CV-467 RLW, 2019 WL 1207866, (E.D. Mo. Mar. 14, 2019), a retail pharmacy brought claims against Express Scripts, alleging Unfair Competition, breaches of contract, breaches of the implied covenant of good faith and fair dealing, interference with economic advantage, violation of uniform trade secrets act and fraud for the practice of “slamming,” that is, collecting claims information received by the PBM at the point-of-sale from retail pharmacies submitting claims for their patients, and providing that same data to Express Scripts’ wholly-owned mail order pharmacy for the purpose of soliciting the same patients to receive their prescriptions via mail order. The core of all the claims was Express Scripts’ conduct of collecting and using prescription data to boost its mail-order operations. Parsing the “black letter” language of the one-sided contract of adhesion, the Judge, however, held that the conduct was not prohibited and, in fact, was expressly allowed under the terms of the agreement with the pharmacies. While the Eighth Circuit revised the standard slightly as it relates to the pharmacy provider’s rights under HIPAA, the Court of Appeals ultimately upheld the lower court’s decision, serving as a reminder of the unbridled power that the PBMs believe themselves to hold.
7.3 What Can Be Done?
Prescription trolling and patient slamming is perhaps one of the most deceitful of the PBM tactics and requires a response at many levels to end it once and for all:
- Legislative
-
- Congress should enact federal legislation which would protect patient choice of pharmacy and prohibit PBMs from requiring patients to use the mail order and specialty pharmacies they own, creating a conflict of interest, or exploiting private patient data for those purposes.
- State lawmakers should enact anti-steering laws like Louisiana’s or Georgia’s, which prohibit PBMs from directly or indirectly steering patients to a pharmacy in which the PBM maintains an ownership interest or control.
- Regulatory
-
- The Office of Civil Rights (OCR) should pursue complaints against PBMs and PBM-owned pharmacies for misappropriation of PHI for pecuniary gain and seek fines as well as injunctive relief.
- State Boards of Pharmacy should pursue complaints against PBMs and PBM-owned pharmacies for violations of Pharmacy Practice Acts, including anti-patient steering laws.
- State Departments of Insurance should pursue complaints against PBMs and health insurers for violations of Any Willing Provider laws, stemming from efforts to deny patients the right to receive care at the pharmacy provider of their choice.
- Plan Sponsor Action
-
- Plan sponsors should negotiate PBM contract terms to require adherence to state laws and CMS guidance.
- Plan sponsors should demand that protections be given for physician-dispensed oncology medications.
Low-Ball Reimbursement
Low-ball reimbursement – when PBMs reimburse providers less than the cost of the drug – is yet another tactic taken by PBMs to effectively exclude community oncology practices, in order to retain and ensure a higher market share for the specialty drug market for their fully owned specialty pharmacies. Also known as “below water” or “underwater” reimbursement, PBMs intentionally lowball the reimbursement rates offered in one-sided, take-it-or-leave-it agreements with providers. No negotiation is offered. The ultimate goal of low-ball reimbursement is to allow the PBM to have it both ways: nominally “comply” with Any Willing Provider laws by “offering” open participation in the network, but in reality, effectively excluding pharmacy providers by pushing them to reject these unsustainable reimbursement rates, thereby diverting more patients to their wholly-owned or affiliated specialty pharmacies. While guised as a cost saving measure, PBMs actually profit off the low-ball reimbursements. As complex, multifaceted health care entities, PBMs are able to recoup any losses that might be incurred at the dispensing level by charging plan sponsors more money through spread pricing (see, Section 4, supra) or receiving rebates or other “fees” from manufacturers at the PBM level (see, Section 3, supra).
This recently played out in the wake of the collaboration agreement between Prime Therapeutics and Express Scripts, causing low-ball, below water reimbursement for community oncology practices. On April 1, 2020, Prime Therapeutics began applying Express Scripts’ lower reimbursement rates and pharmacies have been receiving abhorrently low, even negative, reimbursements. Claims specifically for lifesaving medications and limited distribution drugs are rendered below water. Notably, in June 2020, Blue Cross Blue Shield of Alabama (recognizing that these rates may not be sustainable) began increasing rates to independent pharmacies in Alabama for Blue Cross Blue Shield Alabama plans (however, this plan was the exception to the rule). Many community oncology practices continue to face unsustainable, below cost reimbursement, which is only exacerbated when taking into account direct costs associated with pharmacy operations (such as salaries and benefits of pharmacy staff, accreditation fees, shipping, dispensing fees, supplies and equipment, license fee, pharmacy dispensing software fees and adherence and symptom management software fee, postage, etc.), and indirect overhead (including rent, utilities and telephone charges).
With the impact that this has across the industry, a question is often asked: how are PBMs able to do this? The answer is simple: their excessive market power enables them to unilaterally dictate reimbursement rates where pharmacy providers have essentially no choice but to accept them. As noted above (see, Section 3, supra), over 80% of the covered lives in the United States are controlled by just five PBMs. In some markets, a single PBM could cover over 85% of the patients seen by a community oncology practice. As a result of this concentration, and the inability of patients to freely select their PBM (see, Section 3, supra), being in network with each PBM network is critical.
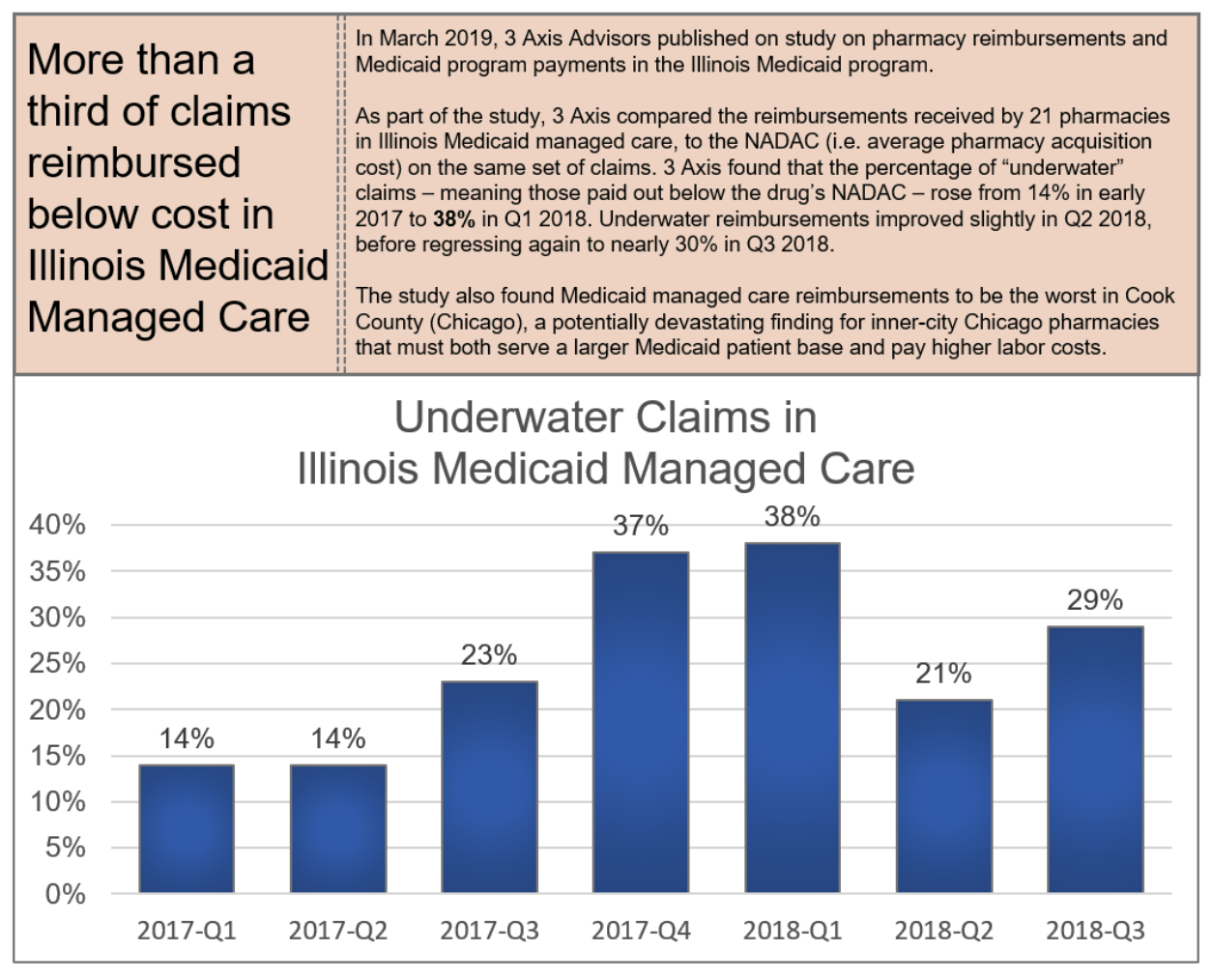
8.1 Who is Impacted?
Ultimately, the substantial and unreasonable reduction in reimbursements creates a provider “desert,” making it impossible for them to stay in business because market share is shifted to PBMs. This turns patients into “hot potatoes” who are passed between different providers because no provider wants to fill medications at losses of hundreds of dollars, with scant guarantee of whether any of these downward prices are actually being passed on to plan sponsors. As vertically integrated models enable PBMs to dominate the pharmaceutical supply chain, community oncology practices are often forced to accept reimbursement below cost because patients have no other choice but to participate in a plan that chooses to use one of these PBMs to manage its pharmacy benefit. Ultimately, low-ball reimbursement harms the provider of choice for the patient, which in turns harms the well-being of patients.
8.1.1 Harm to Patients
As a result of low-ball reimbursements, patients are often forced to receive care only from pharmacy providers owned by or affiliated with PBMs, replete with conflicts of interest between patient care and costs of service. This has had disastrous consequences.
For one, it is well-established that provider participation in pharmacy networks will be decreased as a result of low-ball reimbursement, leaving patients with fewer choices for care. This, in turn, will lead to worse overall care (see, Section 6, supra).
Worse yet, this has the possibility of turning patients into “hot potatoes,” where even contracted specialty pharmacies (including ones owned by or affiliated with PBMs) refuse to fill a patient’s prescription and risk losing money. Sadly, this was the experience of many patients in the immediate wake of the Express Scripts-Prime Therapeutics collaboration. In one particular example involving a Blue Cross Blue Shield of Alabama beneficiary (whose benefits processed under Prime Therapeutics), a provider attempted to fill a prescription for one of its patients but was unable to because of the unsustainable loss the below water reimbursement would have. Consequently, the provider had to attempt to transfer the patient’s prescription to at least four different specialty pharmacies (including several PBM-owned or affiliated pharmacies), in order to finally find a pharmacy that was able to fill the medication (i.e., had access to the limited distribution drug), was contracted with the payer to be reimbursed for the prescription (i.e., held the Blue Cross Blue Shield Alabama Oncology Specialty Network contract), and was willing to accept the reimbursement (i.e., take a substantial loss on the prescription). After trying multiple pharmacies in four states, the patient was finally able to get their medication from a specialty pharmacy located several states away. The whole process took almost two weeks to fill the medication for the patient, causing the patient to run out of her life-saving medication.
These low-ball reimbursement practices have not been limited to commercial plans. As yet another example of patients being “hot potatoes” with no regard for their well-being, within the TRICARE program, which was established by statute to provide health benefits coverage to active duty and retired military service members and their dependents, community oncology practices have reported per-fill losses of $500.00 on every prescription for Imbruvica (an oral oncolytic used to treat certain lymphomas and leukemias), $525.00 on every prescription for Jafaki (a common oral oncolytic used to treat certain bone marrow disorders), and $740.00 on every prescription for Alecensa (an oral oncolytic used to treat lung cancer). Community oncology practices have reported that over eighty percent of their TRICARE claims reimburse at or below cost, while those that reimburse above cost generally have a margin of less than one percent. As a result, this has caused veterans to become “hot potatoes” passed between pharmacy providers (even by PBM-owned or affiliated pharmacies), who are unwilling to fill the medication at a loss.
8.1.2 Harm to Plan Sponsors
As noted, any so-called benefits or savings are nebulous at best. In reality, vertically-integrated PBMs are able to take a “loss” at the pharmacy level, and make up for it by overcharging the plan sponsor. The anticompetitive nature of low-ball reimbursements further allows PBMs to receive “off invoice” discounts and manufacturer payments that help offset the low and under water reimbursement rates at the pharmacy level. For example, PBM-owned or affiliated pharmacies can be willing to nominally “accept” the same reimbursement terms applicable to other pharmacy providers, but they are able to recoup those “losses” by either obtaining discounts from the manufacturer in drug purchases (which are not passed through to the plan sponsor), or simply utilizing spread pricing which is where the PBM charges the plan sponsor an amount much higher than what is paid to the provider and pocketing the profits, or the “spread,” for itself (see, Section 10, infra). In a recent examples, patients and providers have studied Explanations of Benefits (EOBs) and identified instances where a PBM or health insurance company issued, in essence, two separate EOBs for the same claim: one to the provider and one to the patient. The EOBs transmitted to the provider showed the actual amounts being paid, while the one to the patient made it appear as though a much larger amount was being paid by the plan sponsor to the provider. In reality, PBM was simply keeping the difference. Thus, PBMs are using the plan sponsor’s money to profit from driving independent pharmacy providers out of the marketplace. Ultimately, the fact that plan sponsors will not experience increased savings will lead to fewer pharmacy providers in the network, making it more difficult for plan sponsors to get fair terms in the future.
8.1.3 Harm to Providers
The harm of low-ball reimbursement to community oncology practices is self-evident. Each day, more and more community pharmacy providers go out of business due to negative margins as a result of reimbursements below the acquisition and dispensing costs of the prescriptions they provide to patients. Providers often times are not able to pick and choose which rates they will accept and which ones they will not. As a result, if providers challenge low-ball reimbursement at the initial contracting stage, PBMs will likely exclude the provider from the network. For community oncology practices, that means they would be unable to dispense oral chemotherapy to patients. Likewise, when providers have raised concerns about unsustainable reimbursement rates after agreeing to participate, they risk being immediately and summarily terminated without cause.
For practices that choose to stay and accept the low-ball reimbursement rates, they experience a reduction in the ability to provide enhanced services and coordinate patient care, as a direct result of the underwater reimbursements. And when combined with the heightened credentialing standards necessary to even seek admission to these networks, providers face a veritable Catch-22 of having to choose between undertaking the high costs and extra workload of becoming accredited in order to participate in the network, only to then become unable to afford to perform the required services because of low reimbursement once admitted.
8.2 What Does the Law Say?
As in the case of restrictive networks and unreasonable barriers of entry (see, Section 6, supra), federal and state Any Willing Provider laws can offer protection against low-ball reimbursement to the extent they require PBMs to offer participation on “reasonable” and “relevant” terms and conditions. In this regard, as it relates to the federal Any Willing Provider law, CMS expressly recognized that unreasonably low reimbursement terms, which would include below water reimbursements, violate the federal Any Willing Provider law. This serves as a strong rebuke to low-ball reimbursement in the Medicare Part D space.
Recognizing this as a growing problem in the private commercial insurance sector, many states have passed “Fair Price Laws.” For example, the recently enacted New Jersey law, codified at N.J.S.A. 17b:27f-1 to -10, provide PBM pricing transparency and strengthen the rights of pharmacies to contest below-cost reimbursement. Likewise, Arkansas law prohibits PBMs from setting the price for certain generic medications below available pharmacy acquisition costs.
Several unfair trade and unfair competition laws may also be implicated by a PBM’s conduct of setting below water reimbursement to increase market share for its wholly-owned or affiliated specialty pharmacy. For example, under California’s Unfair Competition Law (UCL), Section 1702 of the California Business and Professions Code, known as the “Unfair Competition Law” or “UCL,” “any person who engages, has engaged, or proposes to engage in unfair competition may be enjoined in any court of competent jurisdiction.”
Finally, to the extent such PBM’s low-ball reimbursement is deemed to be seeking monopolization, Section II of the Sherman Antitrust Act may be implicated as well. The Sherman Act provides that it is unlawful to “monopolize, or attempt to monopolize … any part of the trade or commerce among the several states, or with foreign nations.” And further, in the context of state-level UCL claims, conduct may also be deemed to be “unfair” under the UCL if it is “conduct that threatens an incipient violation of an antitrust law, or violates the policy or spirit of one of those laws because its effects are comparable to or the same as a violation of the law, or otherwise significantly threatens or harms competition.”
8.3 What Can Be Done?
Low-ball reimbursement has the potential to fundamentally and irreparably impact our health care system for years to come, and requires action at many levels:
- Legislative
-
- Congress should enact federal legislation extending Medicare’s Any Willing Provider requirements to the TRICARE program, requiring that terms and conditions be reasonable and relevant, and allow for private enforcement of these requirements.
- States should enact Any Willing Provider Laws (where none currently exist) or amend existing Any Willing Provider laws to require that health insurance companies and PBMs allow all pharmacy providers (including community oncology practices) the right to participate in pharmacy networks based on “reasonable and relevant” terms and conditions, applicable to other similarly situated participating providers.
- States should enact laws, like New Jersey’s Fair Price law, requiring PBM pricing transparency and prohibiting below-cost reimbursement to pharmacies.
- Regulatory
-
- CMS should pursue complaints against Part D plan sponsors and contracted PBMs for unreasonably low reimbursement in violation of the federal Any Willing Provider Law and the Medicare Part D Drug Benefit Manual, seeking fines, Warning Letters, and injunctive relief.
- CMS should issue regulation providing “guard rails” on what constitutes reasonable and relevant terms and conditions, and clarify that whether given terms are “reasonable” or “relevant” can be adjudicated in a private contractual dispute between Part D plan sponsors/PBMs and pharmacies.
- State Departments of Insurance should pursue complaints against PBMs and health insurers for violations of Any Willing Provider laws, stemming from efforts to constructively deny providers the right to participate in pharmacy networks based on unreasonably low, below cost reimbursement rates.
Mandatory White Bagging for Cancer Medications
A growing – and extremely concerning – trend that has emerged is the concept of mandatory “white bagging” of oncology medications that are administered in-office by community oncology practices.
“White bagging” occurs where a physician writes and orders a particular medication for an in-office procedure, and rather than being sourced from the physician’s medication inventory, a separate specialty pharmacy fills a prescription, and delivers the drug directly to the prescriber or clinic who retains the medication until the patient arrives at their office for administration.
Likewise, “brown bagging,” which is less common, involves a similar concept, except that instead of causing the prescription to be delivered directly to the community oncology practice, the specialty pharmacy dispenses the medication to the patient him or herself, who then brings the medications into their physicians’ offices for administration in those settings.
In seeming unison, several health insurance companies (who coincidentally have integrated PBMs and specialty pharmacies) have begun to mandate that certain intravenous (IV) medications that were previously purchased by practices and administered in-office to patients, are now requiring that they be filled by the PBM-owned or affiliated specialty pharmacy through white or brown bagging. These are medications that historically have been administered in-office by community oncology practices and billed to patients’ medical benefit (as opposed to their pharmacy benefit). Because these are IV medications, they cannot be self-administered by the patient, and still need to be infused by a health care provider. In essence, these payers (which include Anthem Blue Cross of California, Blue Cross Blue Shield of Tennessee, and Cigna) have mandated that cancer patients receive their chemotherapy through white or brown bagging, to be supplied by the payers’ affiliated specialty pharmacy.
Each of these scenarios present immense concerns for patients, plan sponsors and providers alike. Community oncology practices note that white or brown bagging disrupts the chain of control of expensive cancer drugs; risking improper storage and handling of toxic substances; can unnecessarily cause delays in the onset of treatment; create waste when dosages are changed to, for example, manage adverse events; and places an administrative and liability burden on both patients with cancer and their oncologists.
9.1 Who is Impacted?
9.1.1 Harm to Patients
Patients stand to suffer the greatest as a result of payer and PBM mandatory white or brown bagging policies. Unlike instances where the community oncology practice sources the medication from its own inventory, the physician has no control over the sourcing, storage, preparation, or handling of the specialty oncology medications in white or brown bagging situations, and as a result, patients are exposed to potentially serious harm. The community oncology practice cannot guarantee the integrity and legitimacy of the products being provided by the PBM-owned or affiliated pharmacy, especially as it relates to the shipment and delivery from the specialty pharmacy to the practice. “The difficulties that white bagging policies place on cancer patients are a prime example of the potential harm.”
When medications do not follow the typical chain of custody, the integrity and safety of the medication cannot be guaranteed. When a community oncology practice sources a medication from its wholesaler to be infused in a patient, the community oncology provider is given a Transaction Report or “T3” that details every single transaction involving that medication, going all the way up to the manufacturer that made it. This ensures proper pedigree at each stage along the way. When the practice receives the drug as a white bag from a PBM-owned specialty pharmacy, it is not provided with that information. Worse yet, it has no control or insight into how the specialty pharmacy is handling that product, or how it ensured stability and integrity during the delivery process. This provides risks for patients receiving medications of unknown integrity, where chain of custody cannot be guaranteed.
Patients also stand to be impacted by excessive delays and unnecessary burdens from white bagging when forced to receive their cancer and related treatments from PBM-owned or affiliated pharmacies (as compared to when the community oncology practice sources products from its own inventory for in-office administration). Delays in receiving the medication past an anticipated date are commonly caused by a variety of factors, including failed delivery, incorrect medications being delivered, medications shipped to the wrong address, prior authorization issues, out of stock medications, etc. When medications are sourced from the community oncology practice, issues such as drug shortages can be identified right away, and adjustments made. Requiring that the prescription be sent to and filled by a PBM-owned or affiliated specialty pharmacy can cause confusion and the potential for missed treatment doses.
Finally, patients may be subject to higher out-of-pocket liability when prescriptions are “white bagged” for in-office administration. In addition to having to pay the copayment or coinsurance for the administration procedure, patients will also be responsible for a separate copayment from the pharmacy associated with the dispensed drug product. Required use of the PBM-owned or affiliated specialty pharmacy means that “reimbursement comes not from a patient’s medical benefit but from the pharmacy benefit, and that can mean higher out-of-pocket costs for patients,” as pharmacy benefit copays are typically higher than copays under the medical benefit. Moreover, because PBM-owned or affiliated pharmacies will require patients to have paid for drugs before they are shipped, this can interrupt critical treatment if patients cannot afford to pay for the therapies (a problem that is only exacerbated if the PBM-owned or affiliated pharmacy does not assist the patient in qualifying for payment assistance programs to help meet their cost-sharing obligations, which few do).
Alternatively, even when everything goes “smoothly,” waste can result if extenuating life circumstances cause a treatment plan to be adjusted or an appointment to be rescheduled and the pre-provided “white bagged” medication will not still be good by the time the appointment is rescheduled. This would not occur if the community oncology practice were able to simply source the medication from its own inventory at the time of the patient’s visit.
9.1.2 Harm to Plan Sponsors
The greatest harm to health care payers stemming from mandatory white bagging is in the form of excess drug waste. When a physician utilizes drugs the community oncology practice has on hand in its inventory, the physician is able to quickly and efficiently address patient care real time and avoid waste. Oncology regimens are complex and often require dosing adjustments at the time of administration or therapy cancellation depending on the patient’s laboratory results, scans, and other clinical considerations, such as shifts in the patient’s weight. When utilizing medications from the onsite inventory, physicians are able to make these changes at the time of administration without any delays or risk of waste (they can simply select a different medication or dose off the shelf). However, the same cannot be said if the medications are supplied by PBM-owned or affiliated specialty pharmacies.
Under white bagging mandates, the physician is required to write a “prescription” and send it to the PBM’s wholly-owned or affiliated specialty pharmacy to be filled. Circumstances requiring dosing adjustments or therapy cancellation could occur in the time between when an “order” is written by the physician, and when the medication is received from a specialty pharmacy. Moreover, once the prescription has a patient-specific label, it cannot be returned to stock, unlike products kept within the practice’s inventory for in-office administration. As a result, the entire medication would essentially go to waste, costing the plan sponsor and patient potentially thousands of dollars.
Moreover, plan sponsors face a great risk of being double billed when PBM-owned or affiliated pharmacies bill separately for the drug product, while community oncology practices bill for the procedures and supplies associated with in-office administration. When a community oncology practice submits a claim to an insurer for in-office administration of a drug to its patient, it typically submits a CPT Code for the professional services associated with the administration (e.g., CPT 96413), as well as a J-Code for the medication (e.g., J9271 in the case of Keytruda). CPT Code 96413 corresponds with “Chemotherapy administration, intravenous infusion technique, up to one hour, single or initial substance.” Thus, when submitting claims in this manner, the physician receives his or her fee for the professional services associated with mixing the drug and administering it to the patient but is also reimbursed for the costs of the medication, the diluents, the supplies, the tubing, as well as the associated overhead.
At the same time, when the PBM-owned or affiliated specialty pharmacy uses an NDC number to bill the patient’s PBM, the pharmacy may also be billing (and receiving reimbursement) for overlapping products/services (which it is not actually providing or performing). Many PBM contracts prohibit pharmacies from dispensing medications in their unfinished form, and prohibit billing medications that require reconstitution (e.g., injectable medications) as compounds (suggesting that reimbursement for the diluent and other supplies necessary for administration are included within the total payment).
In addition, many PBMs pay a “dispensing fee” on all claims in addition to the reimbursement for the drug, which is intended to cover costs that are incurred at the point of sale in excess of the ingredient cost of the drug, including the “measurement or mixing of the drug,” “filling the container,” physically providing the completed prescription to the patient, “delivery,” “special packaging,” “salaries of [workers],” “costs associated with maintaining the [ ] facility and acquiring and maintaining technology and equipment necessary to operate the [ ] facility.” While the wholly-owned or affiliated specialty pharmacy that is white bagging will be selecting the product, processing the claim, and causing delivery to the practice, many of these items for which the wholly-owned or affiliated specialty pharmacy will be receiving reimbursement are actually tasks that will ultimately be completed by the community oncology practice. The community oncology practice will continue to be responsible for mixing the drug, procuring the diluent and other necessary supplies, and physically administering the medication to the patient. Thus, this has the risk of the wholly-owned or affiliated specialty pharmacy being paid by the patient’s PBM for the same services that are also being reimbursed by the plan sponsor to the community oncology practice (and which in fact are being performed and provided by the practice).
9.1.3 Harm to Providers
Finally, the greatest harm to community oncology practices stemming from mandated white bagging are increased, unfunded administrative burdens, along with increased legal liability which the providers have no choice but to accept. Community oncology practices are faced with increased administrative burdens as they are expected to undertake all work associated with preparing, diluting, and administering the drug, without being able to seek reimbursement for the medication itself. When medications are white bagged, they typically come in the original manufacturer vials. Apart from the added burdens of storing the products and maintaining them in a separate inventory (since they are patient-specific), in order to be administered to the patient, the products must also be mixed by the practice’s staff and placed into a bag to be infused intravenously. In many instances, IV chemotherapy products are combined with other drug products, as physicians often order a “cocktail” of different drugs and therapies that must be taken in concert. Community oncology practices have to perform these services, despite the fact that they are not being reimbursed for the drug itself. This burden is only exacerbated when the physician makes changes or amendments to the treatment, often after the prescription has been written, but closer in time to when the patient is receiving care. Because the prescription has already been filled and provided by the specialty pharmacy, the practice’s staff must engage in extra work to remedy the problem.
In addition, and more concerningly, community oncology practices face additional liability for their part in prescribing and administering drugs received from outside pharmacies. In October 2012, 64 people died and over 700 people became sick as a result of contaminated compounded steroid injections supplied by New England Compounding Center (NECC). The medications had been ordered by physicians for in-office administration to their patients in clinics and surgery centers. However, due to insanitary conditions at the pharmacy, several batches of the medications had become tainted with fungus, causing many patients to develop fungal meningitis and become seriously ill or die. In the wake of this, dozens of lawsuits (including multiple class actions) were filed against not only the pharmacy, but also the clinics, surgery centers and underlying physicians. Under current white bagging mandates, community oncology practices are forced to accept this additional risk and exposure, as “the primary onus for patient safety remains with providers despite [PBMs and] health plans stripping those providers of their control over the quality and handling of drug therapies.” With white bagging, practices no longer control the acquisition of these medications, and as drug therapies become more complex, thereby requiring additional resources and focus in storing, mixing, compounding and administering the products, they are bearing an inappropriate share of the risks.
9.2 What Does the Law Say?
In April 2018, the National Association of Boards of Pharmacy issued a report entitled “White and Brown Bagging: Emerging Practices, Emerging Regulation”. The report concluded that while “the terms and conditions of this business model are most often set by third-party payers”, issues regarding authenticity and integrity of the drug and adverse patient outcomes are left to the state boards of pharmacy to grapple with in an effort to protect the public. As such, some state boards (e.g., Massachusetts) have specifically prohibited these practices, under various provisions such as “re-dispensing of medication” or handling hazardous drugs.
On the state level, several state legislatures have either prohibited or allowed white and brown bagging practices. For example, Texas, Minnesota, and New York (Medicaid) have prohibited one or both of these practices. Other states like California, have laws that require health plans to demonstrate that their medical decisions are “unhindered by fiscal and administrative management.”
At the same time, many states’ laws may bear directly on arrangements mandating that community oncology practices write prescriptions and send them to PBM-designated specialty pharmacies. For example, many states have “Anti-Patient Steering” laws, which generally prohibit health care providers from agreeing to prescriptions to a particular pharmacy. As an example, New Jersey law provides that “[i]t shall be unlawful for a pharmacist to enter into an arrangement with a health care practitioner, or any institution, facility or entity that provides health care services, for the purposes of directing or diverting patients to or from a specified pharmacy or restraining in any way a patient’s freedom of choice to select a pharmacy.” As another example, Georgia law likewise specifically prohibits pharmacies from presenting (and prohibits pharmacy benefits managers from paying) claims for reimbursement that were received pursuant to a referral from an affiliated PBM.
9.3 What Can Be Done?
Mandatory white bagging harms both patients and plans sponsors, while increasing liability to community oncology practices, and requires a response at many levels:
- Legislative
-
- States should enact laws prohibiting payer-mandated white bagging for community oncology practices and allow patients to receive their in-office oncology medications from their treating oncologist.
- Regulatory
-
- State Boards of Pharmacy should adopt regulations requiring pharmacies that fill prescriptions for white bagging obtain written consent from the physician’s office prior to dispensing the medication, and have policies and procedures in place that (i) track and assure security and accuracy of delivery for dispensed prescriptions until they are administered to the patient; (ii) provide for counseling to patients who are administered white bagged products; (iii) address the return of any prescription medications not delivered or administered to the patient; (iv) assure the confidentiality of patient information; (v) obtain consent from the patient for using such a delivery process through white bagging; and (vi) provide lowest number of vials wherever possible, so as to avoid excess closed-system-transfer requirements and potential USP <800> exposures.
- Practical Considerations
-
- Pharmacies providing white bagged medication should be required to assume all liability associated with the applicable medications/prescriptions and defend/indemnify health care providers who accept white bagged medications.
- Plan Sponsor Action
-
- Plan sponsors should demand that health plans allow patients to continue to receive administered IV chemotherapy medication provided by their community oncology practice of choice.
Spread Pricing and Middleman Profits
Spread pricing occurs when PBMs charge plan sponsors one price for the cost of a patient’s drug, while on the other side of the transaction, reimbursing the dispensing community oncology practice or pharmacy at a lower rate, while pocketing the difference, or the “spread,” for themselves. It is the classic case of the middleman mark up, but played out in a massive and extraordinarily opaque scale. This practice has recently come to light in the Medicaid context, where PBMs manage benefits for state Medicaid MCOs, and where state governments have uncovered immense spreads in drug claims for Medicaid beneficiaries. Ultimately, spread pricing practices reveal how PBMs are vertically integrated enterprises that control vast swathes of the drug supply chain create an anti-competitive marketplace, ultimately driving up the cost of drugs to public health programs and, ultimately, to patients themselves.
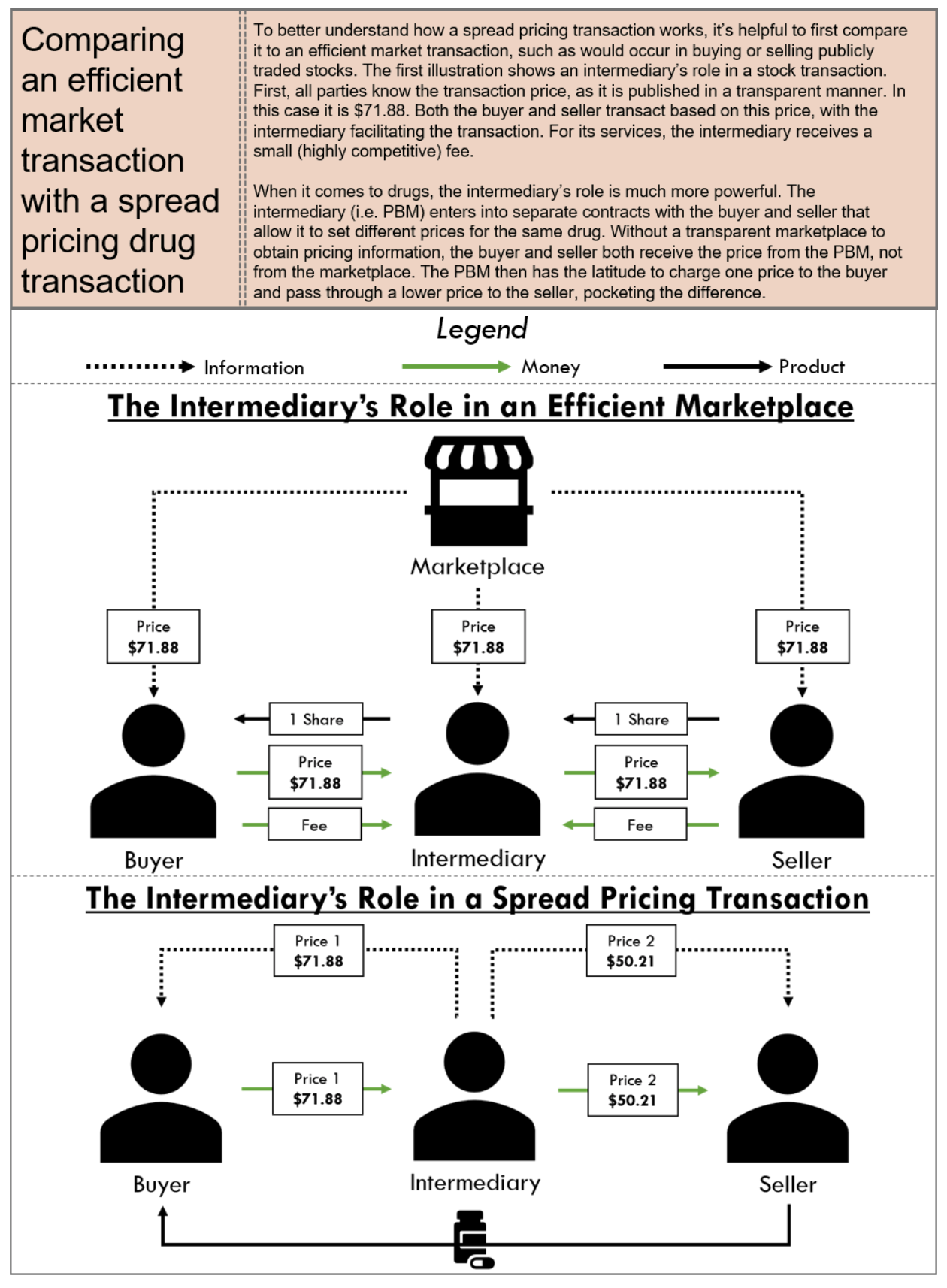
10.1 Who is Impacted?
10.1.1 Harm to Patients
Spread pricing harms patients by increasing premiums and drug prices. As with many other PBM pricing strategies, spread pricing has the perverse tendency to drive drug prices up as the higher the overall drug cost is, the greater opportunity for the PBM to earn a larger spread. In addition, because pricing strategies put in place by PBMs that are not equitable or uniform across different drugs, and perverse financial incentives can be created, putting patients at risk of having pharmacy providers prioritize certain patients with certain disease states over others based on the arbitrary profitability that a PBM applies to the therapy. Finally, in the context of generic drugs, where patients expect to realize the greatest pricing relief, spread pricing artificially increases the cost of such drugs, thus negating such price relief.
10.1.2 Harm to Plan Sponsors
Plan sponsors, and in particular, state Medicaid programs, have been immensely harmed in the inflated prices they – and ultimately the taxpayers – have paid to PBMs because of spread pricing. Ohio was one of the first states to audit PBMs after a Columbus Dispatch exposé revealed the extent of spread pricing in the state’s Medicaid program. Shortly after the news broke, the Ohio Department of Medicaid released a summary of its spread pricing analysis which showed PBMs grabbing $223.7 million in hidden pricing spreads within the Medicaid managed care program from Q2 2017 to Q1 2018, accounting for 8.8% of overall (pre-rebate) spending on prescription drugs.
The Ohio revelations have led to other states and the federal government investigating spread pricing practices within their states, as well as independent efforts. State government work in Kentucky, Georgia, Virginia, and Maryland has definitively quantified spread in their states’ Medicaid programs, while 3Axis Advisors – an independent pharmaceutical policy think tank – has uncovered evidence of spread pricing in New York, Illinois, Michigan and, notably, a 200-page report on spread pricing in the Florida Medicaid program.
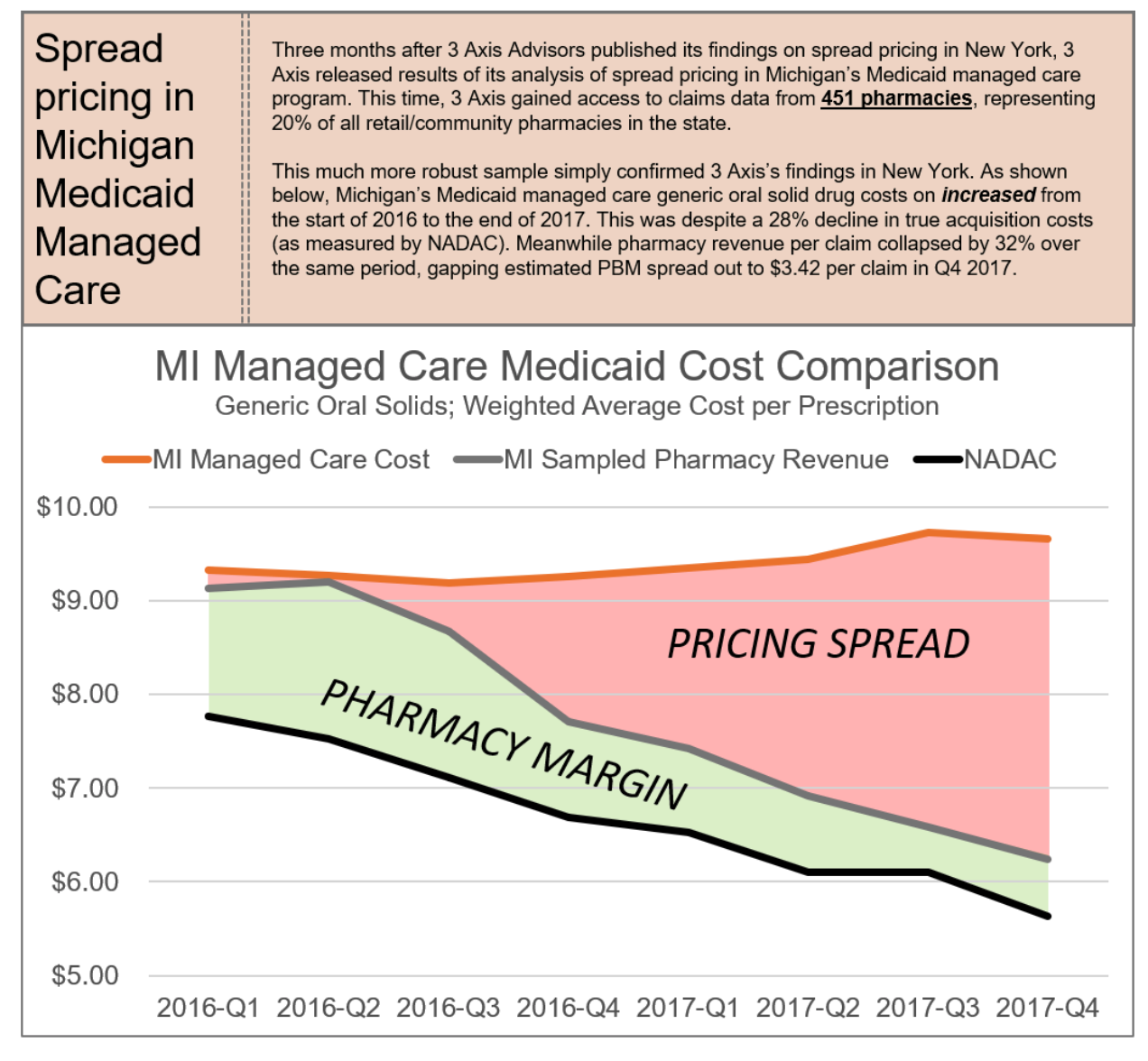
10.1.3 Harm to Providers
Finally, spread pricing has a direct impact on providers, who rely on adequate reimbursement to serve Medicaid patients. In Ohio, the same state to expose $223.7 million in excess charges through spread pricing, many independent pharmacies were reporting such severe loses on Medicaid prescriptions that it made it virtually impossible to continue to participate in the program. The exposure of these abuses led Ohio Medicaid to require certain PBMs, including CVS Caremark, to increase the amount of reimbursements being paid to independent providers (who up until that point, were pocketing the immense spreads).
10.2 What Does the Law Say?
Given the perverse impact of spread pricing upon patients, payers, and providers, CMS’ Medicaid and Children’s Health Insurance Program (CHIP) managed care’s final rule adopted standards for the calculation of Medical Loss Ratios (MLRs). More specifically, the final rule clarified that spread pricing must be reported and included in the calculation of MLRs, which represents the percent of premium revenue that goes toward actual claims and activities that improve health care quality, as opposed to administrative costs and profits. CMS regulations require Medicaid and CHIP managed care plans to report a MLR and use and MLR target of 85 percent in developing rates.
A number of states have implemented measures to prevent PBMs from utilizing spread pricing schemes when contracting with state Medicaid managed care plans. For example, Ohio Medicaid directed its five managed care plans to terminate contracts with PBMs with spread pricing model and enter into new contracts with PBMs with transparent “pass-through” model in 2018. In similar vein, Nevada has enacted transparency bill specifying that a PBM has a fiduciary duty to a third party that contracts with the PBM for pharmacy benefit management services and must notify the third party in writing of any activity, policy, or practice of the PBM that creates a conflict of interest that interferes with the PBM’s ability to discharge its fiduciary duty. New York is also planning to no longer use PBMs and instead, to use fee for service to pay for its prescription drugs.
10.3 What Can Be Done?
The practice of spread pricing by PBMs has recently become an area of focus for plan sponsors seeking to reign in PBM abuses and reduce costs. Potential solutions to spread pricing include:
- Legislative
-
- Congress should enact federal legislation that would require pass-through pricing for covered outpatient drug prescriptions in Medicare Part D and in Medicaid (including managed care).
- States should enact laws like the Nevada law requiring PBMs to be fiduciaries to plan sponsors (i.e., PBMs must act in the plan sponsors’ interests) and providing plan sponsors with a cause of action against PBMs if they utilize opaque pricing not in the plan sponsors’ best interest or favor the PBMs’ wholly-owned or affiliated pharmacies over independent pharmacies or community oncology practices, if this would ultimately be detrimental to the plan sponsors.
- State should enact laws requiring PBMs to report drug costs charged to and paid by plan sponsors and disclosure of such reports to providers.
- Regulatory
-
- Like in Ohio, state regulators should take immediate action, where such action is permitted under enabling statutes, to prevent state Medicaid plans from contracting with PBMs using spread pricing methodology.
- The FTC should enhance oversight and revise antitrust guidance defining impermissible vertical integration structures which could, at the very least, curb the most blatant PBM anti-competitive behavior.
- Plan Sponsor Action
-
- Plan sponsors should implement robust Request for Proposal procedure to select transparent PBMs.
- Plan sponsors should review and negotiate transparent contract terms including, without limitation, an exclusive pricing benchmark.
- Plan sponsors should require PBMs to provide reporting of reimbursements paid to the pharmacies on pharmacy claims and the corresponding charges made to the plan sponsor.
Copay Accumulators and Maximizers
The increased prevalence of high deductible health plans or plans involving patient coinsurance has left more and more Americans finding themselves with significant annual out-of-pocket copayments, coinsurance obligations or deductibles for their medications. Many patients struggle to meet their deductible and pay the copays for the high-cost drugs they need to treat serious, sometimes life-threatening, illnesses like cancer. In a study published in the Journal of Clinical Oncology, the researchers found that drug abandonment and adherence problems are increasingly prevalent in patients prescribed an oral cancer medication due to higher out-of-pocket costs. To help offset these costs – especially in the oncology space, where copayments can range in the thousands of dollars – many drug manufacturers have created copay discount cards to reduce the net out-of-pocket amount to a figure that is affordable to many patients.
However, beginning in 2018, several large insurance companies and PBMs began to implement a nefarious new set of schemes called “copay accumulator programs.” Copay accumulator programs restrict manufacturer contributions to copay discount cards from being applied to patients’ annual deductibles and out-of-pocket maximums. Normally, the contributions from the drug manufacturer’s copay card would not only help offset the patient’s copay at the point-of-sale but would count toward fulfilling the patient’s out-of-pocket obligations (i.e., the deductible). Thus, after several fills of a high-cost specialty medication, the deductible would be exhausted, and the patient’s out-of-pocket would be lowered to an affordable amount. This is important, because many drug manufacturers’ copay coupon programs have annual limits or caps, preventing patients from receiving unlimited copayment assistance. Without copay accumulator programs, patients are able to afford their prescriptions throughout the whole year.
Conversely, when a copay accumulator program is implemented, the amounts of the patient’s copay that have been funded by a drug company (through a copay coupon program) no longer count towards the patient’s out-of-pocket limits. The result is that, after the patient exhausts the benefits from the manufacturer’s copay coupon program, the patient is still left with excessively high copayment obligations.
The financial impact of copay accumulator programs is demonstrated well in an example. Consider an example where a patient is prescribed a drug that costs $36,000 per year, or $3,000 per month. The patient obtains a copay coupon card from the drug’s manufacturer, with an assistance limit of $12,000 per year. The patient’s benefit plan has a $3,000 deductible and, after the deductible has been met, a monthly copay of $500. Without the copay accumulator program, the drug manufacturer would cover the $3,000 deductible in month one (January), and $500 per month each month thereafter. The patient would never run out of benefits under the copay coupon program, and would never be saddled with excessive out-of-pocket costs, significantly reducing the risk of therapy abandonment.
With the copay accumulator program in place, however, the patient would use the copay coupon to cover the monthly drug costs in months one through four (i.e., January through April), and would have no out-of-pocket expenses during those first four months of the year. However, because the copay accumulator program would prevent the amounts received through the coupon from applying toward cost-sharing requirements, the patient would still be required to pay the full deductible amount ($3,000) in month five (May), and monthly copays of $500 per month thereafter. In essence, the maximum benefits under the copay coupon program would have been exhausted at the end of April (having funded $3,000 per month). Here, when the patient is now saddled with a $3,000 bill to continue therapy he or she has been on for four months, there is tremendous risk of therapy abandonment.
These programs have been called a variety of things by different entities, including “Out-of-Pocket Protection Programs” (Express Scripts), “True Accumulation” (CVS Caremark), and “Coupon Adjustment: Benefit Plan Protection Program” (UnitedHealthcare). However, the main thrust has been to place financial roadblock in the way of patients receiving necessary care, with dubious savings being realized by plan sponsors.
Another related concept that has emerged in response to the negative patient impact from accumulators is that of “copay maximizer programs.” Like copay accumulator programs, copay maximizer programs are designed to allow payers to “extract the full value of the manufacturer’s copay support,” but in reality, swap the “financial cliff” that the patients face under accumulator programs, in favor of a slow and steady drain of resources, without any marked benefits to the patient.
For example, assume again a situation where a patient is prescribed a drug that costs $36,000 per year, and there is a manufacturer-sponsored copay coupon with an assistance limit of $12,000 per year (or $1,000 per month). In the context of a copay maximizer, the patient will still have a deductible of $3,000, but instead of a standard copay, the plan will set the monthly copay to slightly more than the coupon’s value, to, say, $1,200 per month. Each month, the patient will be responsible for $200 out-of-pocket (the difference between what is covered by the copay coupon and the set copay amount).
Worse yet, to the extent maximizer programs do actually deliver copay savings to the patient, it invariably comes with underhanded restrictions, obligating the patient to obtain the prescription exclusively from the PBM-owned or affiliated pharmacy, and allowing PBM subsidiaries to reap additional revenue. PBMs have created “secretive and independent private companies” to operate these specialty drug maximizer programs, who sometimes take fees equal to 25% of the manufacturer’s copay support program.
In each of these scenarios, however, the patient is either forced to go over the “financial cliff” in the middle of the year (when their copays skyrocket) and risk drug abandonment, or is forced to utilize a PBM-owned or affiliated pharmacy with limited real financial benefits (or face exorbitant out-of-pocket costs).
11.1 Who is Impacted?
Copay accumulator and maximizer programs have clear negative impact on all stakeholders.
11.1.1 Harm to Patients
The harm of copay accumulators and maximizer programs is felt most acutely by patients – especially cancer patients. Unlike instances where there might be lower cost generics available to be used as alternatives when a brand manufacturer’s copay coupon benefits expire, there are no alternatives for the high-priced oncology medications, and when manufacturer copay coupon programs run out as a result of copay accumulator programs, “the individuals who need assistance the most will be unable to receive it, and will end up paying more for their treatments.”
“This poses an adverse impact on adherence to medication regimens, especially when a support mechanism is not in place.” Studies have shown that patients impacted by copay accumulator programs fill their prescription 1.5 fewer times than patients who are not impacted. More critically, data has shown that patients impacted by copay accumulator programs have experienced a 13% drop in adherence – that is, they’ve fallen off therapy – between months 3 and month 4 of a plan year (coinciding with when they reach the annual cap for manufacturer-sponsored copay coupon programs). This is significant as over 75% of impacted patients have said that their adherence will suffer as a result of these programs.
These findings and observations of direct patient harm have been backed up by literature. In a study published in the American Journal of Managed Care, the authors found that after the implementation of copay accumulator programs, Health Savings Account patients on certain high-cost specialty drugs had “significantly lower monthly fill rates, higher risk of discontinuation, and lower [percentage of days covered],” suggesting that copay accumulator programs have “the potential to negatively affect specialty drug use.” This rings true in the cancer context as well. According to a study in the Journal of Clinical Oncology, nearly half of patients with cancer abandon their prescriptions when out-of-pocket costs reach $2,000. Nonadherence can have dire consequences to patients, and accounts for 10% of hospitalizations and 125,000 deaths each year.
Perhaps the best evidence of patient harm is the stories from the patients themselves. In one instance, a nurse case manager from Ohio with multiple sclerosis had long managed her disease with medications, and was able to afford them through copay coupon programs. However, in May 2018, she discovered that her health plan had instated a copay accumulator program, that required her to pay $3,600 per month for her prescription drugs until she met an $8,800 deductible, forcing her to consider rationing her medication that allowed her to function in her daily life. In another well-publicized incident, a 27-year-old hemophilia patient had been able to afford the $38,000 for his maintenance drugs with the assistance of manufacturer copay coupon programs. However, once his health plan instituted a copay accumulator program, he was unable to afford the $6,350 deductible. As a result of his immediate and unforeseen inability to afford the medications, he was left with untreated bleeds, resulting in internal bleeding, and needing additional surgeries to correct. “The patient has been in and out of the hospital, is currently in a wheelchair, and is not working, all at a cost of $3.5 million.”
One of perhaps the most sinister aspects of copay accumulator and maximizer programs for patients is the overall lack of transparency. These programs lack any semblance of transparency, and are “often implemented without a patient’s knowledge or full understanding of their new ‘benefit.’”
Ultimately, because patient receiving medications that have lower-cost generic products have the ability to switch to such generic products in the face of copay accumulator and maximizer programs, it is the sickest patients requiring the highest-priced drugs that are most egregiously affected by these programs, and are in essence “subsidizing the patients who are adequately served by lower-cost pharmaceuticals that have low or no copays.”
11.1.2 Harm to Plan Sponsors
When the PBMs created and rolled out copay accumulator programs, they were billed as a cost savings tool for plans sponsors, such as employers. In theory, it does make sense when applied to high-cost branded medications, when a lower-cost, equally effective generic product is available. These programs counteract manufacturer efforts to retain market share for brand drugs once generics have become available, and further the interests of pushing patients to lower cost alternatives. However, in the oncology space, cancer care is for life saving treatment and does not have the same risks of “overutilization,” nor are there cheaper alternatives available.
Instead, the result of copay accumulator or maximizer programs is that the harms and additional costs to plan sponsors caused by drug abandonment and non-adherence will far outweigh any potential savings to be gained from them. From increased hospitalizations, additional treatments, and more catastrophic care, it is well-established that plan sponsors save money when patients stay adherent to the drugs they are prescribed. This is especially true in the cancer context, where studies have suggested that the increased plan costs caused by non-adherence due to copay accumulator programs was more than double than that of all other disease groups.
Worse yet, many employers and plan sponsors do not even know what they are getting or whether such programs have been instituted. While nearly 20% of commercial medical insurance policies sold in 2018 will have copay accumulator/maximizer programs built in, “most employers who have purchased/are purchasing these plans are unaware these programs are present in the coverage” and “have no idea how it will adversely affect their employees’ care.” This is especially alarming considering the secretive operations of copay maximizer programs, where the prescription is typically required to be filled at the PBM-owned or affiliated pharmacy, and related or affiliated companies take up to 25% of the copayment assistance made available by the manufacturer.
For example, with Express Scripts’ SaveonSP program, a commercial plan sponsor declares specialty drugs to be “non-essential health benefits,” making them covered by the plan, but not subject to out-of-pocket maximums mandated by the Affordable Care Act. In turn, the patients’ out-of-pocket costs are set to the maximum annual value of a manufacturer’s copay coupon program. “For instance, a program with a total value of $20,000 in copayment support would require a patient to pay $20,000 annually for their drugs, without regard to the plan’s out-of-pocket maximums.” Thereafter, to avoid these inflated costs, the beneficiaries must enroll separately in the SaveonSP program, and have their prescriptions filled exclusively by Express Scripts’ Accredo specialty pharmacy. SaveonSP then charges a fee equal to 25% of the copayment support, or $5,000 in the above example. This is in addition to the profit generated by Express Scripts’ wholly-owned pharmacy by filling the prescription.
Ultimately, while copay accumulator and maximizer programs might seem like a good short term solution, the devil is in the details, and in reality, these programs will ultimately increase costs for plan sponsors in the long run, including increased hospitalizations, additional care, and overall increases to drug prices.
11.1.3 Harm to Providers
Finally, community oncology practices are harmed by manipulative copay accumulator and maximizer practices as well. When physicians prescribe a particular oncology treatment to be dispensed out of the community oncology practice, they undertake a “difficulty and time-consuming process” involved in finding financial assistance for their patients. This includes finding manufacturer-sponsored copay coupon programs, providing resources to patients, and potentially providing supporting documentation to these programs. The copay accumulator and maximizer programs will add additional complexities in the patient coverage process and will only increase “the administrative burden on practice staff, who will now need to understand the nuances of co-pay accumulators and maximizers; as well as help explain to patients why some of the assistance is not helping them to reach their deductible.”
In addition, community oncology practices are further impacted when their patients discontinue prescribed therapy due to cost. Many community oncology practices are contracted with payers under value-based arrangements, where they take responsibility – and sometimes risk – for the outcomes of patients. If a patient stops taking his or her therapy once the copay coupon program is exhausted, that patient may wind up in the hospital or needing additional care. This will in turn negatively impact community oncology practices’ performance under value-based contracts.
11.2 What Does the Law Say?
Federal statutory law is silent on the issue of copay accumulator and maximizer programs.
However, in 2019, HHS finalized the Notice of Benefit and Payment Parameters for 2020 (NBPP 2020), which only allowed health plans to implement copay accumulator programs when both a brand and generic medication were available. In essence, this would have allowed plans to steer patients to less costly, generic medications when possible, but would provide protections for patients – including cancer patients – who did not have access to alternative, less costly medications.
However, on May 7, 2020, HHS released its Notice of Benefit and Payment Parameters for 2021 Final Rule, which clarified certain confusion created by different agencies’ guidance, and now allows health plans to implement copay accumulator programs regardless of whether or not a generic alternative is available. When patients cannot afford their medications, they may rely on copay assistance (i.e., coupon cards from drug manufacturers). These coupon cards not only contribute toward the patient’s copay but also count toward the patient’s annual deductible. Thus, as of July 30, 2020, HHS has not only allowed health plans to implement these programs but has removed key protections for cancer patients.
Fortunately, however, several states have enacted their own laws governing copay accumulators (importantly, in NBPP 2021, HHS explicitly stated that the Final Rule does not preempt state laws that govern the use of copay accumulator programs in state-regulated health plans). At this time, four states (Illinois, West Virginia, Virginia, and Arizona) have enacted copay accumulator legislation. While these apply to state-regulated plans (and not to Exchange-based health plans), they provide protection against certain PBM conduct.
For example, Virginia Statute § 38.2-3407.20 requires health plans to include any amount paid by or on behalf of a plan enrollee when calculating an enrollee’s overall contribution to any out-of-pocket maximum or any cost-sharing requirement to the extent permitted by federal law and regulation. Likewise, in Illinois, 215 Ill. Comp. Stat. Ann. 134/30 requires health plans to apply any contributions (i.e., third-party payments, financial assistance, discount, product vouchers, or any other reduction in out-of-pocket expenses) for prescription drugs made by or on behalf of an enrollee toward that person’s deductible, copay, or cost-sharing responsibility, or out-of-pocket maximum.
An additional eight states have some form of legislation pending to address copay accumulator/maximizer programs.
11.3 What Can Be Done?
- Legislative
- State should enact laws that require health plans to include any amount paid by or on behalf of a plan enrollee when calculating an enrollee’s overall contribution to any out-of-pocket maximum or any cost-sharing requirement.
- Regulatory
-
- HHS should rescind the Notice of Benefit and Payment Parameters for 2021 Final Rule, and institute Notice of Benefit and Payment Parameters that, at the very least, reinstates, strengthens, and clarifies the protections for patients receiving medications without lower cost alternatives.
- Plan Sponsor Action
-
- Plan sponsors should inquire with PBM whether copay accumulator and/or maximizer programs are being employed, and demand that protections be given for oncology medications that lack lower cost alternatives.
Maximum Allowable Cost (MAC) Pricing
Maximum Allowable Cost pricing, or “MAC,” is one of the most significant and challenging issues facing independent pharmacies throughout the United States today. While not as impactful to community oncology practices providing cancer care as many of the other topics addressed in this exposé, MAC has nevertheless become one of the most manipulated and opaque methods by which PBMs control reimbursement to independent pharmacies and has become a catalyst for legislative efforts to rein in PBM conduct.
MAC is typically defined as the maximum amount of money that PBM will pay a pharmacy for certain multi-source drugs, typically multi-source generic drugs. It is well established that generic drugs make up the vast majority of drugs dispensed throughout the United States. For example, according to the Association for Accessible Medicines, generic drugs account for approximately 89% of all prescription drugs dispensed in the United States. Thus, generic drugs constitute the majority of drugs dispensed to patients throughout the United States meaning MAC pricing present a significant issue as it pertains to provider reimbursement.
MAC began as a mechanism to save money in health care and incentivize selective and intelligent purchasing practices, but MAC has since evolved over time into a PBM tool that can be manipulated by PBMs to increase revenues in several different ways. MAC pricing is a PBM created pricing benchmark – MAC prices and MAC lists are prepared exclusively by PBMs and considered by the PBMs to be proprietary and confidential. Moreover, PBM-set MAC rates need not have any relationship to a drug’s market clearing acquisition cost. As such, the creation and publication of PBM MAC prices and MAC lists are shielded from the public and avoid public scrutiny.
PBMs’ ability to keep MAC lists and MAC prices from the public has enabled PBMs to utilize MAC pricing to increase their revenues and to effectuate certain PBM practices that lead to higher revenues, including the PBM practice of spread pricing, wherein a PBM reimburses a pharmacy provider one price for a drug but collects a higher amount from the plan sponsor and retains the difference. The fact that MAC pricing is shrouded in secrecy, and there is no requirement for MAC rates to have any basis in real costs, creates substantial profit opportunities for PBMs and has resulted in substantial challenges for independent pharmacy providers over the past several years.
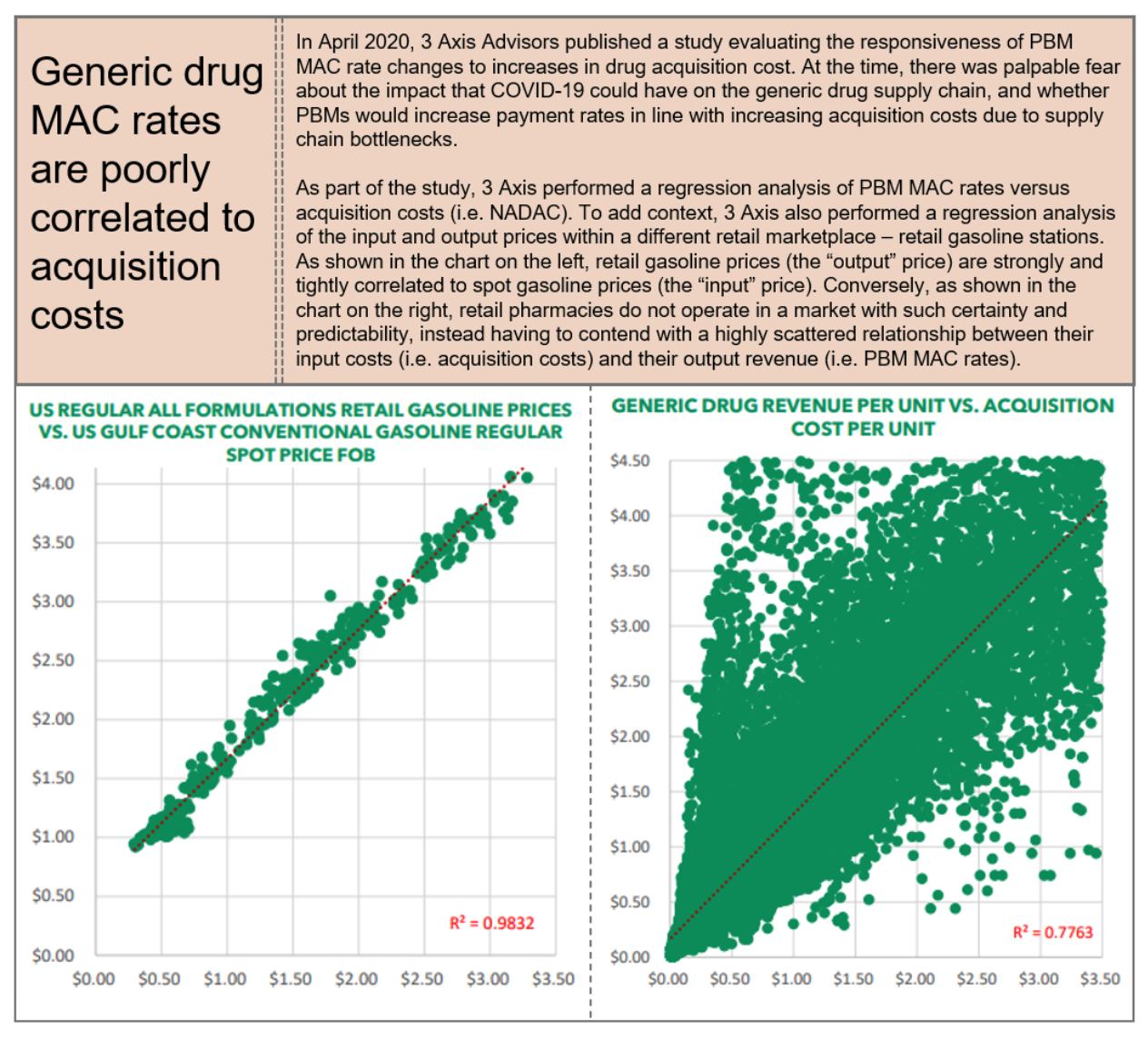
12.1 Who is Impacted?
PBMs’ secretive MAC pricing tactics have caused harm to payers, providers, and most critically to patients.
12.1.1 Harm to Patients
The improper use of MAC pricing tactics harm patients throughout the United States by limiting patient care access – this specific issue is on display in the case Rutledge v. PCMA which successfully went before the Supreme Court of the United States in December of 2020. In Rutledge, Arkansas enacted a law, Act 900, with the purpose of addressing this patient-based issue, which was especially pronounced in rural areas. MAC pricing appears to often disproportionately harm patients in rural areas, who often do not have access to a broad catalogue of different (sometimes cheaper) products, thereby harming these patients specifically. “MAC methodologies are resulting in pharmacies closing down, especially in rural areas . . . [and] approximately 44% of Arkansans live in rural areas.” The potential for patient harm based upon improper pricing and reimbursement tactics, including MAC pricing combined with spread pricing was also discussed at length in the Pennsylvania Auditor General’s Report on PBMs, wherein it was noted that “small pharmacies often see the most vulnerable patients . . . [a]nd if small pharmacies are forced out of business, these patients will have to travel greater distances to get the medications they need[.]” Thus, there is ample objective evidence that PBMs’ MAC pricing tactics are causing harm to patient populations throughout the United States and that this harm may be particularly pronounced in rural settings.
12.1.2 Harm to Plan Sponsors
In addition to harming patients, improper MAC pricing tactics by PBMs also potentially harm all payers including Medicare, Medicaid, employers, and taxpayers, although studies indicate this may be particularly pronounced in the Medicaid context. “PBMs’ control of MAC definitions allows them to manipulate the MAC concept in whatever ways they choose.” Thus, MAC lists do not afford payers and sponsors with the ability to have predictability or in any way guarantee savings but instead give PBMs unfettered discretion to control precisely which drugs are on a particular MAC list and to ensure only those drugs which they are making money on remain on the list and those which they are not are removed from the list. In assessing potential harm of PBMs’ MAC pricing tactics, it is important to note that MAC pricing applies to drugs, most commonly generics, and not to specific programs (e.g., Medicaid. The implication is that improper MAC pricing tactics can affect all payers, including federal and state governments, and by extension, taxpayers.
As mentioned, MAC pricing is one of the primary methods by which PBM spread pricing is effectuated, wherein the PBM bills a plan sponsor one price and reimburses the pharmacy provider a lower amount. Several studies have shown that improper MAC pricing tactics, in connection with spread pricing, has been prominent in the Medicaid context, including in Florida, Georgia, Illinois, Kentucky, Maryland, Michigan, New York, Ohio, and Virginia. Pennsylvania’s report on PBMs noted that in 2017 “three PBMs made between $2 million and nearly $40 million on spread pricing, earning average profits between 28 cents and almost $13 per Medicaid prescription filled.
In order to implement spread pricing via MAC pricing, PBMs create numerous different MAC lists including separate MAC lists for each individual payer as well as separate MAC lists for PBM network providers which enables a PBM to bill a plan sponsor one rate but pay the pharmacy a separate and frequently a much lower rate. Florida, Michigan, New York, and Ohio are three states that exemplify the harm caused by PBM MAC pricing tactics to state specific Medicaid programs.
12.1.3 Harm to Providers
Finally, improper MAC pricing tactics by PBMs also harm providers due to unsustainable reimbursement by PBMs. In Rutledge, the District Court acknowledged that numerous pharmacies had been harmed by these tactics and were closing down, noting that “[i]ndependent community pharmacies have had to eliminate employees during the last five to ten years due to the financial hardships they have faced.” The court further noted that “[i]ndependent community pharmacies in Arkansas are in economic distress.”
These unreasonably low MAC prices are further exacerbated by the fact that PBMs are often slow to make price adjustments to MAC drugs when there are market conditions that would result in the PBM reimbursing a higher amount (i.e., an increase in acquisition cost), but relatively quick to make price adjustments based on market conditions that would result in the PBM reimbursing a lesser amount (i.e., a decrease in acquisition cost).
12.2 What Does the Law Say?
Given its prevalence in commercial insurance contracts and Medicaid programs, MAC pricing has largely been regulated by the states. Currently, 36 states have some form of MAC law or MAC appeal law in place. While the different state laws vary in the level of protections they afford to pharmacies regarding MAC, there are several general characteristics in these state MAC laws. Typically, robust MAC laws will establish criteria for placing a drug on a MAC list, establish an appeal process for challenging questionable MAC pricing, and set requirements for updating MAC lists. Texas and Georgia are example of such laws. In Texas, a PBM may not include a drug on a MAC list unless: (1) the drug: (A) has an “A” or “B” rating in the most recent version of the United States FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, also known as the Orange Book; or (B) is rated “NR” or “NA” or has a similar rating by a nationally recognized reference; and (2) the drug is: (A) generally available for purchase by pharmacists and pharmacies in [Texas] from a national or regional wholesaler; and (B) not obsolete. Further, the PBM must develop a process for pharmacies to appeal MAC prices of a drug on or before the 10th day after the claim is submitted and the PBM must respond within 10 days.
Texas’ MAC appeal requirements also require that when an appeal is successful, the PBM must (1) adjust the MAC that is the subject of the appeal effective on the day after the date the appeal is decided; (2) apply the adjusted MAC price to all similarly situated pharmacies as determined by the PBM; and (3) allow the pharmacy that succeeded in the appeal to reverse and rebill the pharmacy benefit claim giving rise to the appeal. When appeals are not successful, the PBM must identify and disclose (1) each reason the appeal was denied; and (2) the NDC number from the national/regional wholesalers from which the drug is generally available for purchase by pharmacies in Texas at the MAC price that is the subject of the appeal. Moreover, in Texas, there are separate guidelines governing MAC in the Medicaid context. Although there are some functions an MCO may delegate to a PBM in Texas, there are also certain functions for which an MCO is ultimately responsible despite the delegable nature of the function. These expressly include “negotiation and establishment of pharmacy provider reimbursement rates [and] cultivation and maintenance of MAC pricing lists.” Thus, certain laws in the Medicaid context potentially provide additional recourse against payers in addition to PBMs as is the case in Texas.
12.3 What Can Be Done?
Effective responses to improper MAC pricing by PBMs require action at various levels:
- Legislative
- Congress should enact legislation at the federal level prohibiting MAC manipulation including a requirement as to transparency in MAC pricing on both sides of the PBM—at the plan sponsor side as well as the provider side.
- States must take more aggressive action against PBMs’ MAC pricing tactics and enact new laws or else enhance existing laws that mandate transparency in reimbursement which should include robust laws that protect both plan sponsors and providers from manipulative MAC pricing practices, especially as it pertains to claims that are paid from taxpayer dollars, e.g., Medicare and Medicaid.
- For legislative efforts to be effective, the laws enacted must provide a deterrent beyond solely relying on government enforcement. Thus, it is imperative that states enact laws or enhance existing laws by including or adding “private rights of action” to ensure plan sponsors and providers have recourse against improper PBM MAC pricing tactics and also make violation of MAC laws by PBMs an unfair or deceptive trade practice act in accordance with existing state law.
- State laws should strive for greater uniformity, including in how MAC is defined to prevent inconsistencies in reimbursement practices throughout the country, greater/broader appeal rights (requiring that the drug utilized as the basis for the MAC rate is readily available and conforms with the state’s prescription substitution laws), and to ensure that PBMs cannot take liberties in placing drugs that do not meet a uniform definition of a MAC drug on a MAC list.
- MAC laws should permit providers to choose how MAC appeals are filed rather than permitting PBMs to force providers to use a pharmacy services administrative organization (PSAO) – this ensures that if PSAOs are not responsive to MAC issues, pharmacy providers can pursue the appeals on their own or hire third parties that may be more effective at addressing MAC issues.
- Regulatory
-
- There should be increased scrutiny over PBM MAC pricing tactics at the federal level through CMS/OIG audits.
- Increased scrutiny over PBM MAC pricing tactics should happen at the state level through audits by both the Departments of Insurance and Departments of Health and/or state Boards of Pharmacy.
- Plan Sponsor Action
-
- Plan sponsors must demand more from PBMs during the contracting process including specific information on how pharmacies are being reimbursed and the use of MAC lists as it pertains to both the plan sponsors’ relationship with the PBM as well as pharmacies’ relationships with the PBM to understand if spread pricing is being used and/or whether pharmacies are being harmed by improper MAC reimbursement.
Effective Rate Reconciliation
In yet another opaque and underhanded ploy, PBMs have created and utilized the concepts of Generic Effective Rate (GER) and Brand Effective Rate (BER) to essentially reprice drugs, and claw back pharmacy reimbursements, sometimes more than a year after drugs are dispensed. GER and BER (collectively known as the “Effective Rate”) measure the discount that the PBM contractually must deliver for its client (i.e., plan sponsors) to a benchmark called Average Wholesale Price (AWP) for generic prescription drugs and for brand-name prescription drugs, respectively. However, because they are assessed retrospectively and on a network level basis, it is tantamount to giving PBMs unbridled discretion as to how they will pay a given pharmacy, and still technically be in compliance with the reimbursement terms of the agreement.
Worse yet, PBM methods of imposing and recouping Effective Rate assessments are equally deceitful. Not only did many PBMs foist such reimbursement terms on pharmacy providers retroactively without their knowledge or consent (for example via retroactive contracts with the pharmacy providers’ PSAOs), but in many instances, the pharmacy providers only learned of the Effective Rate reconciliation when the PBMs, either directly or indirectly, simply began withholding payments due to offset the alleged Effective Rate overpayments.
Because of its after-the-fact assessment applied across an entire network of pharmacy providers, Effective Rates allow PBMs to circumvent Maximum Allowable Cost laws enacted by many states (see, Section 12, supra), and hinders pharmacy providers’ ability to challenge underwater reimbursements on generic prescriptions. At its most basic level, Effective Rate is not reflected at the point of sale and it provides an opportunity for PBMs to take back a substantial amount of reimbursements on prescription drug claims that were already dispensed to patients.
Similarly, PBMs have also created another pricing mechanism called Dispending Fee Effective Rate (DFER) to recoup dispensing fees already paid to providers that provides no purpose to reduce plan sponsors’ drug spending. DFER allows a PBM to pay one dispensing fee at the point-of-sale, and afterwards claw-back a portion of this dispensing fee down to the contractually specified DFER. This particularly pernicious type of effective rate undermines the cost-plus pass-through contracts that many state Medicaid programs are contemplating, or moving to, in response to outrage over spread pricing. DFERs could allow the PBM to pass through the state-mandated dispensing fee, only to claw it back after the fact, without the state’s knowledge.
13.1 Who is Impacted?
13.1.1 Harm to Patients
As with many other PBM tactics, including spread pricing (see, Section 10, supra), rebates (see, Section 4, supra), and DIR fees (see, Section 5, supra), Effective Rate reimbursement frameworks have the ability to increase the gross price for medications, notwithstanding a potentially lower net price. For Medicare Part D patients, Effective Rate forces them to reach “donut hole” and pushes patients into “catastrophic coverage” at a much faster rate. As discussed in detail below, this results in the patients being responsible for a greater share of the costs of the medication.
13.1.2 Harm to Plan Sponsors
While it is billed as a “cost containment” and pricing guarantee to payers, in actuality, Effective Rate reimbursement schemes do little to lower the overall costs of drugs. Effective Rate prices are invariably tied to percentage discounts off of reported AWP (as shown in the graphic on page 78, an inherently unreliable pricing benchmark), enabling PBMs to deliver on savings guarantees, while not actually lowering overall costs (as lower generic and brand-name prescription drug costs for plan sponsors would in turn lower overall revenue for PBMs).
An even more pernicious feature of Effective Rate pricing arrangements is that they provide PBMs with the ability to collect “spread” between what they charge their clients (e.g., employers and plan sponsors) and what they pay their providers (e.g., pharmacies and community oncology practices) without having to put their clients in traditional spread pricing contracts. Instead, PBMs can simply sign one contract with a client guaranteeing, say, an 82% discount to AWP and a different contract with their pharmacy network guaranteeing an 87% discount to AWP. Both contracts are highly confidential, so the “buyer” (the employer) and “seller” (the pharmacy) of drugs does not know what each other are paying/receiving. Even if the employer demands a full pass-through contract, in which no spread is taken off the claim, the PBM will simply pass-through what it charges its client to the pharmacy at the time of the transaction, and then claw the overpayment back at a later time through its effective rate adjustments. At the end of the day, in this hypothetical example the PBM has locked in 5% of AWP for its services, regardless if it collects that up front or months after the transaction.
The value of Effective Rate contracts to the PBM does not end there. That’s because for generic drugs, AWP is designed to do exactly the opposite of what prices should do over time for generic drugs – AWP is designed to increase, not decrease over time. So, in our hypothetical example, the hidden 5% of AWP locked in by the PBM becomes more and more valuable each year to the PBM as AWPs diverge from true generic acquisition costs.
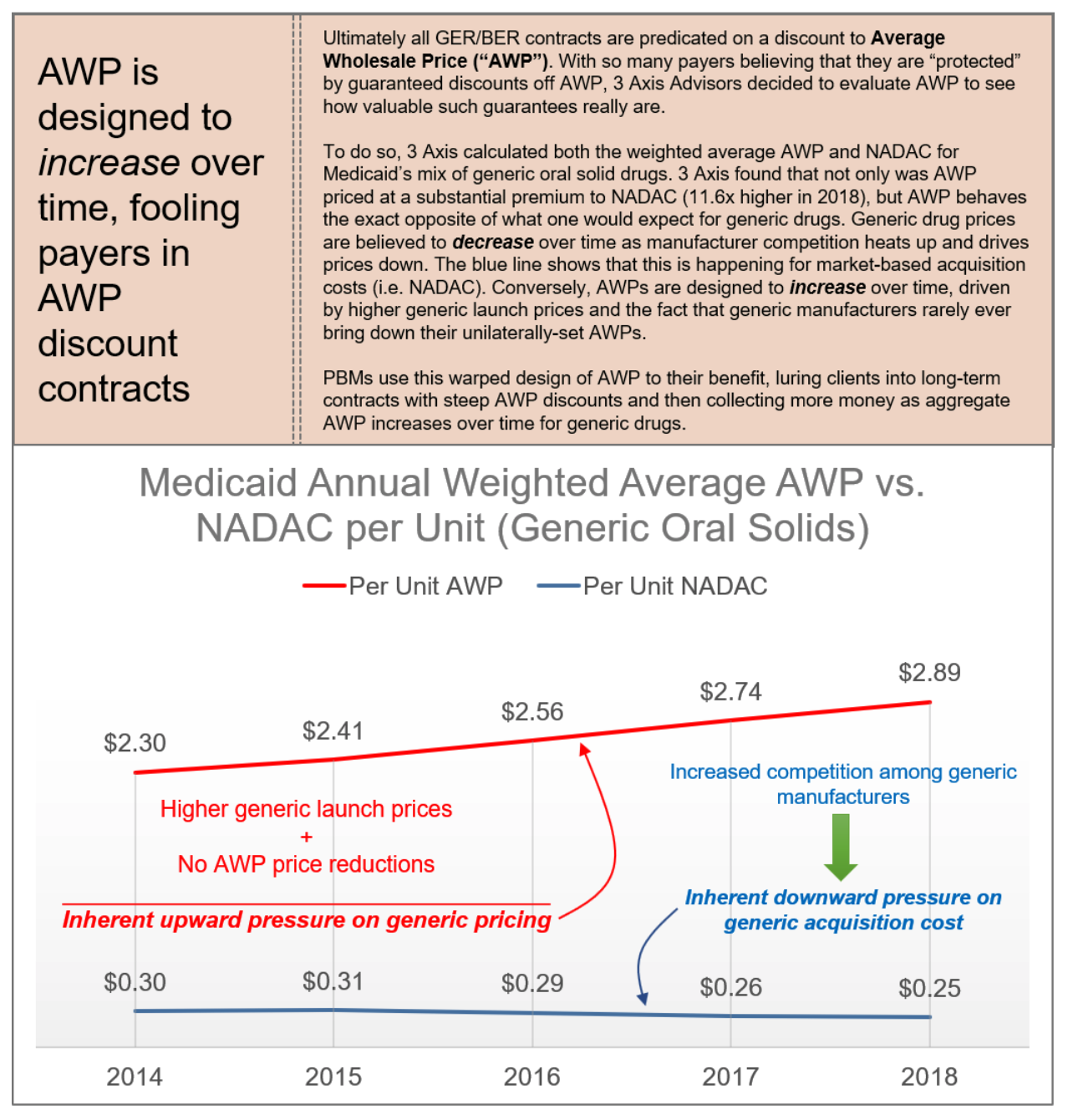
13.1.3 Harm to Providers
As noted above, Effective Rate reimbursement has had an especially damaging impact on providers. By effectively circumventing MAC laws, PBMs are able to reimburse many pharmacies below water on claims, leaving them without any recourse to challenge such reimbursements through legally-mandated appeals processes. This has particularly effect on providers who only dispense a limited range of generic products, such as community oncology practices. PBMs’ reconciliation of Effective Rate is a significant financial hurdle to community oncology practices because oncologists generally treat patients with a handful of drugs compared to other community retail or chain pharmacies who have a broad and diverse patient population.
13.2 What Does the Law Say?
At the federal level, in addition to the guidance on spread pricing generally (see, Section 10, supra), GER and BER reconciliations are properly considered Direct and Indirect Remuneration (DIR), which Medicare Part D plan sponsors must report to CMS. This at least, in theory, requires PBMs and Part D plan sponsors to disclose the extent and amount of GER/BER, regardless of whether it is passed-through to the plan sponsor or retained by the PBM.
At a state level, many states have enacted laws that would prohibit these types of post-point-of-sale reconciliations and clawbacks with respect to private health plans. For example, Tennessee law provides that neither a health insurance company nor a PBM may “charge a pharmacist or a pharmacy a fee related to a claim unless it is apparent at the time of claim processing and is reported on the remittance advice of an adjudicated claim.” Likewise, Indiana law explicitly regulates the practice of “effective rate of reimbursement,” and provides that a PBM may not “[r]educe, directly or indirectly, payment to a pharmacy for pharmacist services to an effective rate of reimbursement…”
Finally, Effective Rate reconciliations may impinge on the multitude of “Prompt Payment” laws that exist in virtually every state in the country. For example, Mississippi’s Pharmacy Benefit Prompt Pay Act requires PBMs to pay electronically submitted claims in full within fifteen days. PBMs’ later-in-time retraction of the amounts paid could violate those requirements.
13.3 What Can Be Done?
Effective Rate reimbursement requires a response at many levels:
- Legislative
- States should enact laws, like Tennessee’s and Indiana’s that prohibit recoupment of fees on claims that were not reflected at the point-of-sale or otherwise ban Effective Rate reimbursement as a construct altogether.
- States should enact MAC Appeal Laws (where none exist) or amend existing MAC laws to prohibit health insurers and PBMs from circumventing MAC appeal rights through Effective Rate reimbursement constructs.
- Laws should be enacted, like New Jersey’s Fair Price law, requiring PBM pricing transparency and prohibiting below-cost reimbursement to pharmacies.
- Regulatory
-
- CMS should audit Part D plan sponsors and contracted PBMs to determine whether GER/BER is appropriately reported and reconciled to CMS at the end of each Plan Year.
- State Departments of Insurance should pursue complaints against PBMs and health insurers for violations of Any Willing Provider Laws, stemming from efforts to constructively deny providers the right to participate in pharmacy networks based on unreasonably low, below cost reimbursement rates.
- Plan Sponsor Action
-
- As part of the PBM contract, plan sponsors should require PBMs to pass through any and all amounts PBMs received from the pharmacies after the point-of-sale on a claim-by-claim level.
- As part of the PBM contract, plan sponsors should require PBMs to seek a permission prior to implementing a contracted-rate with the pharmacies (e.g., GER).
Conclusion
The list of PBM abuses and games is seemingly never-ending and evolving. But the reality is that we are only just scratching the surface of understanding what these abusive health care middlemen are doing. Simply put, PBMs have overwhelmingly abused their responsibility to protect Americans from this country’s drug pricing crisis, instead exploiting the opacity throughout the drug supply chain to enrich themselves. Their many abuses go well beyond just questionable rebate practices, and hurt patients and plan sponsors (including employers, Medicare, and Medicaid).
Unfortunately, their impact is only becoming more pronounced, especially in oncology. More and more cancer drugs are coming out in oral formulations, further shifting care away from the medical space and into the pharmacy space. These expensive therapies are very attractive to PBM’s because of the potential for high prices that yield high rebate revenues, high DIR fees, and eventually, high spreads – all of which are a function of the drug’s cost.
And even outside of the pharmacy benefits realm, through vertical integration, PBMs have been able to exert considerably more influence in the other areas, such as injectable biosimilars and intravenous chemotherapies. Not only can PBMs can leverage these for steep originator and rebates (thereby stifling the biosimilar industry for their own gain), but PBMs have instituted mandatory white bagging policies to take even in-office administration out of the hands of community oncology practices.
The bottom line is this: today’s drug supply chain is designed for cancer patients to receive inferior treatment, while paying more out-of-pocket.
The time for action to stop PBM abuses is now. Each day that goes by, community oncology patients, practices, and professionals become increasingly powerless because of horizonal PBM consolidation and vertical integration with insurers.
Fortunately, however, solutions do exist. These include legislative efforts at both the state and federal levels. Many states’ existing laws serve as prime examples of how they can be successfully implemented to protect the interests of patients and health care payers (like employers, Medicaid programs, and taxpayers). In addition, based on many laws that are currently on the books, regulators (both state and federal) have tremendous tools available to them, that up until this point, have not been widely utilized. The time is critical that regulators – including CMS, OCR, the FTC, state Boards of Pharmacy and state Departments of Insurance – take much need action to rein in the unchecked power of PBMs.
The time for sitting back and letting market forces address the issues is over. The time for action to stop PBM abuses is now.
COA 2022 PBM Dirty Tricks Exposé Report
Executive Summary
There is growing awareness of the problems and pitfalls with Pharmacy Benefit Managers (PBMs) in the United States health care system. Contracted by plan sponsors (including government programs, self-insured employers, and insurance companies) to negotiate on their behalf with pharmaceutical companies, these “middlemen” corporations have quietly become an unavoidable part of our nation’s health care system.
Today, fewer than five PBMs control more than 80 percent of drug benefits for over 260 million Americans, which includes the power to negotiate drug costs, what drugs will be included on plan formularies, and how those drugs are dispensed. Oftentimes, patients are required to receive drugs through PBM-owned or affiliated specialty and mail-order pharmacies and suffer serious, sometimes dangerous, and even deadly, impacts of their abuses as a result of medication delays and denials.
However, while the role PBMs play in the U.S. health care system is complex and under scrutiny by both federal and state policymakers and the public, it is increasingly becoming clear that PBMs make up an oligopoly of rich, vertically integrated conglomerates that routinely prey on health care practices, providers, and their patients. PBMs have done this by overwhelmingly abusing their responsibility to protect Americans from this country’s drug pricing crisis, instead of exploiting the opacity throughout the nation’s drug supply chain to enrich themselves.
Unfortunately, their impact is only becoming more pronounced, especially in the world of cancer care. More and more cancer medications are coming out in oral formulations, resulting in a shift away from the medical benefit and into the pharmacy benefit. And because cancer medications are among the most expensive out there, they are very attractive to PBMs because they yield higher rebates, higher “DIR fees,” and other pricing gimmicks that yield substantial profits.
Through vertical integration and sheer market power, PBMs have also been able to creep into other areas of our health care system, such as injectable biosimilars and intravenous chemotherapies. Not only can PBMs leverage these products for steep originator drug rebates (thereby stifling the biosimilar industry for their own gain), but PBMs have also begun to institute policies such as mandatory “white bagging” to take the in-office administration out of the hands of patients’ oncologists.
The purpose of this exposé is to reveal and explain PBMs’ advantage and leverage by providing transparency where now there is total darkness, and by delving into the many ways that PBMs have abused their power. This report comprehensively explores and documents the myriad of PBM abuses, and their impact on patient care – focusing especially on cancer care. It explores how the recent levels of consolidation among PBMs and health insurers are adversely impacting cancer care, fueling drug costs, all while allowing for massive profits for PBMs and health insurance companies. Examining the most pervasive and abusive PBM tactics, each section highlights the adverse impact of PBMs on patients, healthcare payers (including Medicare, Medicaid, employers, and taxpayers), and providers, while also detailing potential solutions.
Each day that goes by, physicians, practices, and most importantly, patients become increasingly powerless because of horizonal PBM consolidation and vertical integration with insurers. The result is a system designed for patients to receive inferior treatment, while paying more out-of-pocket for their medications.
The time for sitting back and hoping for PBMs to become good faith actors is over. It is time for action to stop PBM abuses once and for all, and this exposé provides a road map for tackling them one dirty PBM trick at a time.
Introduction
In the eyes of many Americans, the problem with drug pricing is caused by unscrupulous pharmaceutical manufacturers who have increased drug prices over the last two decades with reckless abandon. This has been exemplified by a handful of highly visible bad actors, such as “pharma-bro” Martin Shkreli or Nostrum Pharmaceuticals founder, Nirmal Muyle, who rightfully captured the public’s attention, but wrongfully over-simplified the causes of our nation’s drug pricing issues.
Far more dangerous and insidious actors have quietly grown to dominate the nation’s pharmaceutical industry and drive high drug prices through the secretive pharmacy benefit manager (PBM) industry. Ironically, in the country’s attempt to rein in ruthless operators like Shkreli and Muyle, we ended up inadvertently creating the PBM problem that now plagues us. Expanding the role of PBMs, first from simple processors of pharmacy claims to middlemen more actively managing the prescription benefit initially made some sense. Clients – employers, unions, state governments, and other payers of medical care – did not have the expertise to manage complex drug benefits. Thus, they could hire a PBM to administer their prescription benefit, which would include simplifying and streamlining a complicated drug supply chain, designing formularies to exclude wasteful drugs, using their size and leverage to negotiate better discounts from pharmaceutical manufacturers, and managing pharmacy networks to create better outcomes for patients.
However, as this exposé on PBM business tactics, dirty tricks, and their negative impacts will detail, what seemed like a good idea “on paper” has not come to fruition. Instead, the nation’s largest PBMs have capitalized on the complexity of the drug supply chain and used the secrecy in which they operate to hide the true cost of drugs. And rather than eliminate the costly arbitrage within the supply chain, PBMs co-opted and embraced it, exacerbating the very problems of high drug prices that they were originally hired to control. They saw the financial windfall that would come through vertical integration and bought or set up their own mail-order and specialty pharmacies, steering patients away from independent community pharmacies and medical practices to their wholly-owned or affiliated pharmacy facilities where they could retain the inflated prices (and profits) they themselves were responsible for creating.
The perverse result is that PBMs have abandoned their most sacrosanct function of protecting their clients from high cost or low benefit drugs, instead letting higher priced drugs “buy” their way onto their clients’ formularies via rebates that the PBMs mostly retain. They then set up affiliated rebate aggregator entities to further obfuscate the flow of pharmaceutical manufacturer dollars, retaining a larger portion of their clients’ rebates, and leaving patients on high deductible plans exposed to drugs with exploitative list prices. The result is that patients pay more for their drugs off of artificially inflated list prices and the PBM clients have higher prescription drug costs.
The PBM’s purpose in the drug supply chain was to “police” the system. Had the largest PBMs not been lured in by the immense profit potential borne out of the complete opacity of drug costs, a PBM’s greatest asset would have been trust – trust from payers and providers that they were tirelessly working to protect the American public from high drug prices. However, this unfortunately did not come to pass. Instead, the PBM’s greatest advantage has become the almost total opacity of the U.S. drug supply chain and a lack of understanding among employers, unions, state governments, and American taxpayers of how most PBMs have chosen to abuse it.
The purpose of this exposé is to reveal and explain the PBM advantage by providing transparency where now there is total darkness and delving into the many ways that PBMs have abused their power to become “crooked cops.” Throughout this exposé, we comprehensively explore and document the myriad of PBM abuses, and their impact on patient care – focusing especially on cancer care. Finally, we explore how the recent levels of consolidation among PBMs and health insurers is adversely impacting cancer care, fueling drug costs, while allowing for massive profits for PBM and health insurance companies. We have thoroughly examined and detailed the most pervasive and abusive PBM tactics, in each section highlighting their adverse impact on patients, health care payers (including Medicare, Medicaid, employers and taxpayers), and providers.
With the ultimate goal of this exposé being transparency, Frier Levitt went beyond the law, partnering with 3 Axis Advisors LLC to create infographics derived from their analysis of millions of prescription claims across multiples states. The goal of these infographics is to help crystallize and simplify the very complex topics we will discuss throughout this exposé. Lastly, because PBMs have been known to hold themselves out as being “above the law,” we have provided the applicable law and legal principles governing each topic, and detailed the PBMs’ thin legal footing as it comes to these abusive practices. Finally, we have laid out potential, workable solutions to these issues, which may be legislative, regulatory, or legal in nature.
We intend for this report to serve as an authoritative source and reference guide for federal and state policymakers, regulators, and employers seeking greater understanding of PBM behavior, as well as frameworks for reshaping the industry for the better. While not all PBMs engage in these types of practices, or the degree with which they engage in these practices may vary from plan to plan, program to program, state to state, and so on, we believe that a thorough exposure of the blind spots, latitude for abuse, and backwards incentives is essential for any coherent understanding of the inherent flaws within the drug supply chain.
This exposé was commissioned by the Community Oncology Alliance (COA). The findings reflect the independent research of the authors, Frier Levitt, LLC, and does not endorse any product or organization. If this exposé is reproduced, we ask that it be reproduced in its entirety, as pieces taken out of context can be misleading.
Background
3.1 The Stakeholders
Any examination of the PBM industry must necessarily begin with an overview of the relevant stakeholders. These include five major categories of industry participants: (1) plan sponsors, (2) health insurers, (3) patients, (4) manufacturers, (5) providers, and (6) PBMs. Understanding who the major stakeholders are, and their relationship with one another, is paramount.
At the top of the hierarchy are plan sponsors. These include governmental health benefits programs (such as Medicare, Medicaid and TRICARE), employer-sponsored health plans, Taft-Hartley and union welfare plans, and private health insurance companies. These entities sponsor a health benefits plan for their members, beneficiaries or employees, and provide coverage for pharmacy expenses and drug costs (in addition to traditional medical expenses). In the Medicare Part D context, the Centers for Medicare & Medicaid Services (CMS) contracts with private insurance companies that submit bids to become Part D plan sponsors, and CMS in turn subsidizes certain costs associated with the operation of the plans. Likewise, in the Medicaid space, the majority of states operate a managed care model with respect to pharmacy benefits, contracting with Medicaid Managed Care Organizations (MCOs), who in turn, contract with PBMs to administer the pharmacy benefit. Finally, in the private sector, employers either directly or through an insurance company contract with PBMs to administer pharmacy benefits. These employer-sponsored plans may either be fully-insured (meaning the employer hires an insurance company and pays all or part of the premiums on behalf of its employees) or self-insured (meaning the employer bears all of the financial risk with the costs of care). In any case, these plan sponsors bear the ultimate costs of care, and suffer when PBM abuses cause prices to rise or waste to occur. Plan sponsors may or may not hire a health insurance company to help offset the risks associated with the cost of care, and pay premiums on behalf of their beneficiaries. These health insurance companies may in turn be the entity that directly contracts with the PBM for pharmacy care. However, as noted below, the lines have become increasingly blurred between health insurers and PBMs; thus, the key distinction between plan sponsors and health insurers is that the plan sponsors are typically the ultimate financial guarantors of the costs of the health care for their beneficiaries, including not only drug costs but also major medical expenses.
At the other end of the continuum are the patients. Patients include beneficiaries of government sponsored health care programs, as well as the employees (and dependents) of employers sponsoring health plans. They are also uninsured or underinsured individuals who are left to find a way to cover drug costs themselves. In oncology, they are cancer patients needing care from a complex and disjointed health care system. As a group, they not only bear a disproportionate share of the out-of-pocket costs associated with PBM abuses, but also suffer from the inferior care caused by certain PBMs’ tactics of putting profits over patients. These include delays and denials as a result of PBMs’ unnecessary obstacles to care.
On the front line of care are the providers. These include retail, specialty and mail-order pharmacies, and in oncology, community oncology practices. In addition to providing direct medical care, community oncology practices provide in-office and outpatient pharmacy services, which can take two basic forms (depending on applicable state law): dispensing physician practices (i.e., in-office dispensing under a plenary medical license), or oncologist-owned pharmacies (i.e., the oncology practice owns and operates a licensed retail pharmacy within the clinic). These providers contract with PBMs to dispense medication to plan members, and participate in PBM networks. In so doing, they are tasked with providing appropriate care to their patients, while remaining bound to the PBMs who set reimbursement rates and other terms for participation.
While not directly involved in the provision of care, manufacturers are equally part of the continuum and impacted by PBM actions. These include drug and biologic manufacturers, including both brand and generic companies. Manufacturers have had a particular important role in the biosimilar market, becoming captive to PBMs’ rebate traps, and stifling the biosimilar market before it even has a chance to take hold.
The final piece of the puzzle is the PBM. PBMs are third-party administrators of prescription drug programs covered by a plan sponsor. The PBM is primarily responsible for processing and paying prescription drug claims submitted by participating providers on behalf of covered beneficiaries. However, a PBM’s role is not limited to processing and paying prescription drug claims. Rather, PBMs also provide bundled services related to the administration of pharmaceutical benefits, including formulary design, formulary management, negotiation of branded drug rebates, and controlling network access of participating pharmacies. Perhaps most importantly, PBMs often also own and operate their affiliated retail, mail-order and/or specialty pharmacies, and in so doing, directly compete with independent providers participating in PBM networks. They are not just the gatekeepers, but also competitors operating in the same marketplace. This blatant conflict of interest has serious consequences. Finally, as the result of consolidation and vertical integration within the marketplace, virtually all of the major PBMs have merged with, acquired or become acquired by health insurers, greatly blurring the lines between insurer and PBM. As a result, health insurers and PBMs are often referred to jointly as “payers.”
Figure 1. The Pharmacy Benefits Landscape

The Figure 1, above, visually demonstrates the different stakeholders, and their relationship with one another.
3.2 Consolidation of PBMs and Health Insurers, and the Resulting Influence on Recent PBM Actions
PBMs traditionally have played a critical role in the administration of prescription drug programs. However, over the past ten years, the PBM marketplace has transformed considerably. Changes include both horizontal and vertical integration among health insurance companies, PBMs, chain pharmacies, specialty pharmacies, and long-term care pharmacies. As a result, a smaller number of large companies now wield nearly limitless power and influence over the prescription drug market.
Within the PBM marketplace, over 80% of the covered lives in the United States are controlled by only five PBMs. As a result of this concentration, a pharmacy’s access to these five PBM networks is critical. Being out of network with just one PBM (which in some regions, could make up more than 85% of the market), and being unable to obtain reimbursement for claims dispensed to those patients, could make it financially unviable for any community oncology practice to provide dispensing services at all. The lack of competition in the marketplace stems, in large part, from a series of mergers, integrations, and consolidations. These consolidations and integrations are undoubtedly a factor in many abusive PBM practices, ranging from seeking to exclude independent providers, to reimbursement rates that force providers to lose money by filling prescriptions, to outright diversion of patients to the PBMs’ wholly-owned or affiliated pharmacies. The consolidation increases the market power of the top PBMs, which makes this possible.
The breadth of PBM power did not arise overnight. It began with a series of vertical consolidations in which some PBMs acquired pharmacies and other PBMs acquired insurance companies. In 2007, the shareholders of Caremark Rx, one of the nation’s largest PBMs at the time, approved a $26.5 billion takeover of CVS Pharmacy, which effectively created the first vertically integrated retail pharmacy and PBM. Vertical integration of the industry continued in 2011, as Blue Cross Blue Shield of North Carolina, one of Medco’s largest customers, began shifting its PBM business away from Medco to Prime Therapeutics, a PBM that is wholly owned by a group of thirteen Blue Cross plans across the country. In 2012, UnitedHealthcare (United), the nation’s largest insurance company, began migrating the administration of its plans from Medco Health Solutions to OptumRx, United’s wholly-owned PBM.
Consolidation of the PBM and payer space has not been limited to vertical integration. In 2011, two of the nation’s then-largest PBMs – Medco Health Solutions, Inc. and Express Scripts, Inc. – announced a $29 billion merger. After a contentious regulatory approval process, the Federal Trade Commission ultimately approved the merger in 2012.
Thereafter, the industry continued consolidation both horizontally and vertically. In 2013, a regional PBM – SXC Corporation – agreed to buy another regional PBM – Catalyst, Inc. – for $4.4 billion to form a national PBM, known as Catamaran Corp. In July 2015, Catamaran was acquired by United, OptumRx’s parent company, for $12.8 billion. The two PBMs are now integrating operations and operate under one name, OptumRx. In 2015, Rite Aid acquired the PBM EnvisionRx for approximately $2 billion. Later that year, Walgreens announced its intention to acquire Rite Aid and EnvisionRx for $9.4 billion. Also in 2015, Aetna, the nation’s third-largest insurer, announced its intention to acquire Humana, the nation’s fourth-largest insurer, as well as Humana’s wholly-owned PBM, Humana Pharmacy Solutions, for $37 billion. Finally, in 2015, Anthem announced its agreement to buy Cigna (including its PBM arm) for $48 billion, which would result in, yet again, fewer players in the space. However, on July 21, 2016, the Justice Department filed lawsuits to block both the Aetna-Humana and Anthem-Cigna mergers, asserting that the mergers would quash competition, leading to higher prices and reduced benefits.
Figure 2. PBM Mergers and Consolidations in Last Ten Years

Unfortunately, the last five years has only seen this trend of consolidation and integration expand at an exponential rate. In November 2018, CVS Health completed a controversial $69 billion acquisition of Aetna, a managed health care company that specializes in selling traditional and consumer-directed health insurance along with related services including dental, vision, and disability plans. Not to be outdone, in December 2018, health insurer Cigna acquired Express Scripts for $54 billion. Since that time, Cigna and Express Scripts have continued to expand in creative ways. In December 2019. Express Scripts and Prime Therapeutics announced a three-year collaboration agreement, whereby Express Scripts would take over the contracting and administration of the pharmacy benefits for Prime Therapeutics’ members. As a result of the arrangement, Express Scripts will now manage the prescription benefits for more than 100 million Americans.
Figure 3. Vertical Integration of PBMs and Health care Conglomerates

This rapid evolution of the PBM and health insurance industry shows how a limited number of corporations wield an outsized level of control and influence in the prescription drug coverage marketplace. Fewer payers spells harm to patients, especially cancer patients. These integrated companies have greater abilities to control the nature and direction of patients’ care, including what type of care/drugs they receive, from whom they receive it, and in what setting they are treated. The level of PBM intrusion into the care received by patients borders on the practice of medicine by these PBMs and health insurance conglomerates.
Fewer payers also results in harm to plan sponsors, especially employers sponsoring health plans, who have fewer choices based on decreased competition. This hits small employers the hardest, who lack the overall leverage and resources to either demand competitive rebates or restructure entrenched PBM practices.
Fewer payers also exponentially increases the importance of network access for providers. Exclusion from one PBM with a market share of 35% means that the provider loses out on a major portion of the patient population.
Figure 4. Market Share by PBM in U.S. Prescription Benefits Market in 2018

As can be seen in the figure above, consolidation has created merged entities that have oppressive power. This creates a virtual chokehold note only on community oncology practices and pharmacy providers, but on plan sponsors and patients alike. It is through this market dominance that PBMs are able to get away with their abuses. Whether it is outsized rebates and DIR fees fueling drug prices. Whether it is unreasonable barriers to entry, network exclusions, or mandatory white bagging forcing patients to receive inferior service at higher costs. Whether it is employing insidious copay accumulator programs or deceptive pricing and reimbursement techniques. Or worse yet, whether it is essentially practicing medicine, through “fail first” step therapy, prior authorization requirements, or formulary exclusions, many of which favor not the least expensive medication, but the most profitable one for the PBM. Each of these tactics are made possible by the PBMs’ sheer levels of dominance at all levels of the health care continuum. This consolidation has hurt medical care while fueling both drug prices and costs to patients and plan sponsors alike.
While the Federal Trade Commission (FTC) and Department of Justice (DOJ) Antitrust Division recently embarked on a process to rewrite vertical merger guidelines, this effort is seen by many as coming “too little, too late.” Providers, patients, and plan sponsors have long realized that the vertical integration between payer-PBM-provider would spell disaster for quality and freedom of choice. Dramatic and urgent action is necessary to curtail this wide-ranging abuse of power.
Manufacturer Rebates, Rebate Aggregators, and the “Gross-to-Net Bubble”
It is axiomatic to say that the PBM market is highly concentrated, with three companies (i.e., CVS Caremark, Express Scripts, and OptumRx) covering nearly 80 percent of the market or 180 million American lives. As a result, pharmaceutical and biosimilar manufacturers face exceedingly high stakes when negotiating for formulary placement. Among the different sources of revenue, the most prolific by far is in the form of rebates from pharmaceutical manufacturers that PBMs extract in exchange for placing the manufacturer’s product drug on a plan sponsor’s formulary or encouraging utilization of the manufacturer’s drugs. Rebates are mostly used for high-cost brand-name prescription drugs where there are interchangeable products and aim to incentivize PBMs to include pharmaceutical manufacturers’ drugs on plan sponsors’ formularies and to obtain preferred tier placement.
While drug prices are too high, ironically, the growing number and scale of rebates is the primary fuel of today’s high drug prices. The truth is that PBMs have a vested interest to have drug prices remain high and to extract rebates off of these higher prices. PBM formularies tend to favor drugs that offer higher rebates over similar drugs with lower net costs and lower rebates.
Figure 5. Gross-to-Net Bubble

Apart from increasing costs today, these destructive practices will have a long-lasting impact on the future of health care and drug innovation. Traditionally, generic drugs offer significant price relief for brand medications; however, there are an ever-growing subset of medications that are unlikely to ever have a traditional generic alternative. As a result, federal policy was enacted to create eventual competition for these brand products such as the biosimilar pathway. However, the PBMs’ practice of maximizing rebates may effectively neuter the nation’s biosimilar market before it even gets off the ground. Unlike traditional drug products, biologics are unique and complex molecules and represent many of the new breakthrough treatments that have come to market over the past ten years. But with such breakthrough comes extremely high cost. As a result, biosimilars – that is, products that are “highly similar” to the reference biologic – have emerged to provide alternatives and competition in the biologics space. The first biosimilar product in the United States was approved in March 2015 and marketed in September 2015. The greater use of biosimilars has the potential to reduce the overall drug spending, while providing greater clinical options for providers and patients. However, PBMs and biologics manufacturers have erected “rebate walls” that have severely depressed biosimilar development and widespread adoption. According to former FDA Commissioner, Dr. Scott Gottlieb, Americans could have saved more than $4.5 billion in one year alone, if they had bought FDA-approved biosimilars. While the FDA had approved 11 biosimilars through 2018, only three were then being marketed in the U.S. As of January 2022, nearly 32 biosimilars have been approved, while only 29 are currently being marketed. PBM rebates represent a clear and existential threat to the future of the biosimilar marketplace.
As the American public and plan sponsors have become more aware of the nature and extent of rebates, they have begun demanding that all or nearly all rebates negotiated on their behalf be fully reported and passed-through. As a result, PBMs have begun to market themselves as transparent and assert that many of their customers are able to negotiate “pass-through pricing” allowing pharmaceutical manufacturer rebates and other concessions to flow directly to plan sponsors. However, a dangerous new trend has grown exponentially over the last few years through which PBMs seek to “circumvent” these pass-through requirements. PBMs have increasingly “delegated” the collection of manufacturer rebates to “rebate aggregators,” which are often owned by or affiliated with the PBMs, without seeking authorization from plan sponsors and without telling plan sponsors. Sometimes referred to as rebate GPOs, these mysterious entities include Ascent Health Services, a Switzerland-based GPO that Express Scripts launched in 2019, Zinc, a contracting entity launched by CVS Health in the summer of 2020, and Emisar Pharma Services, an Ireland-based entity recently rolled out by OptumRx. Even some of the major PBMs (i.e., the “Big Three” PBMs) sometimes find themselves contracting with other PBMs’ rebate aggregators for the collection of manufacturer rebates (for example, in the case of OptumRx contracting with Express Scripts for purposes of rebate aggregation for public employee plans).
In both the private sector and with respect to government health care programs, the contracts regarding manufacturer rebates (i.e., contracts between PBMs and rebate aggregators, as well as contracts between PBMs/rebate aggregators and pharmaceutical manufacturers) are not readily available to plan sponsors. Moreover, PBMs do not provide plan sponsors access to claim-level rebate information unless demanded through the contracts entered by and between plan sponsors and PBMs.
Within Medicare Part D, Part D Sponsors are required to submit Direct and Indirect Remuneration (DIR) reports to CMS disclosing the total amount of rebates, inclusive of manufacturer rebates, retained by PBMs regardless of whether such rebates were passed to Medicare Part D plan sponsors. And while PBMs and rebate aggregators are obligated to provide, among other things, the aggregate amount and type of rebates, discounts, or price concessions to the plan sponsors (who in turn provide the same to CMS), PBMs and rebate aggregators do not have to provide claims-level information on the actual amounts received on behalf of plan sponsors.
4.1 Who is Impacted?
The deleterious effects of rebates, and the furtive work of rebate aggregators, are felt across the health care spectrum.
4.1.1 Harm to Patients
Whether a patient has insurance or not, rebates serve to increase the overall costs of drugs and out-of-pocket expenditures for patients. With one in four people in the United States having difficulty paying the cost of their prescription medications, the extent of the negative impact of rebates is felt far and wide.
For uninsured patients, the rebates negotiated by a PBM or health insurance company do nothing to lower their out-of-pocket costs. Rebates promote high drug list prices. “Higher drug prices hurt uninsured patients who pay list prices … based on drugs’ list prices.” And because these rebates are received and kept among secretive health care conglomerates, and not shared with providers or other groups, even discount programs like GoodRx do little to help uninsured patients receive savings on the most expensive drugs.
Even for patients with insurance, rebates ultimately increase costs to the patient for the benefit of PBMs and health insurers. At the point of sale, the inflated list prices caused by rebates “hurt … insured patients who pay coinsurance and deductibles based on drugs’ list prices.” Over the past several years, the number of patients on high-deductible health plans has skyrocketed. This has turned the insurance market upside down, causing the relatively small number of sick patients who pay high copays off of inflated list prices to subsize the cost of care for healthy people. In this form of “reverse insurance,” the sickest patients (e.g., those taking expensive cancer medications) generate a large share of manufacturer rebate payments, which in turn are used to “subsidize the premiums for healthier [patients].” This is the opposite of how insurance is supposed to work.
What’s worse, PBMs’ preference of highly-rebated drugs not only increases patients’ out-of-pocket expenses, but also creates unnecessary burdens in receiving appropriate care, even to the point of fatality. PBMs have an incentive to favor high-priced drugs over drugs that are more cost-effective, because rebates are often calculated as a percentage of the manufacturer’s list price. PBMs receive a larger rebate for expensive drugs than they do for ones that may provide better value at lower cost. This can also occur “when a brand drug goes generic under the Hatch-Waxman Amendments, with the first generic version being granted six months of market exclusivity,” and “[i]n exchange for substantial rebates, manufacturers [are given] an exclusive extension of their brand drug, which circumvents Hatch-Waxman and blocks generic competition.” PBMs’ financial motivations often result in more expensive and less efficacious drugs being placed on the drug formulary, which in turn hurts patient care.
Again, PBMs are able to do this because of the sheer levels of market consolidation and integration, which is adversely impacting cancer care and fueling drug costs all in the interests of PBM profits.
4.1.2 Harm to Plan Sponsors
While rebates are intended to lower the “net price” of drugs, thereby reducing costs to plan sponsors (including employers), there are several important ways that PBM rebates increase the costs of drugs for both plan sponsors and patients.
The first way relates to the ability of plan sponsors, especially self-funded employers, to ensure the full amount of rebates are reported and passed through to them by PBMs. As noted above, it is extremely difficult to gauge the true amount of drug manufacturer rebates collected by PBMs, and this is only made more difficult by the advent of rebate aggregators. Unlike in the Medicare Part D program, PBMs typically do not legally owe self-funded employers any reporting on rebates. PBMs employ exceedingly vague and ambiguous contractual terms to recast monies received from manufacturers outside the traditional definition of rebates, which in most cases must be shared with plan sponsors. Rebate administration fees, bona fide service fees, and specialty pharmacy discounts/fees are all forms of money received by PBMs and rebate aggregators which may not be shared with (or even disclosed to) the plan sponsor. These charges serve to increase the overall costs of drugs, while providing no benefit whatsoever to plan sponsors.
And while there might be greater reporting and disclosure obligations in the Medicare Part D and Medicaid programs, the growth of rebate aggregators has created a way for PBMs (or their corporate affiliates) to retain rebates and not share them with plan sponsors. This causes the Part D plan sponsor to become liable to CMS to “true up” any reductions in cost caused by these rebates, despite the fact that the Part D plan sponsor never actually received any rebates. Moreover, studies have shown that PBM rebates extracted from drug manufacturers drive up the drug spending of plan sponsors including Medicare and Medicaid. This is especially draining on already budget-strapped state governments. Since Medicare Part D is financed through general revenues, beneficiary premiums, and state payments for dual-eligible beneficiaries (who received drug coverage under Medicaid prior to 2006), rebates also drive up the drug spending of the participating states and in turn, taxpayers’ financial obligations to support Medicare Part D and Medicaid continues to rise. The total drug spend of a plan sponsor, regardless of whether it is a federal or state governmental program or a self-funded employer, will inevitably increase because PBMs are incentivized to favor expensive drugs that yield high rebates. In some instances, PBMs purposely misclassify generic drugs as brand drugs to charge higher prices to plan sponsors, which ultimately generate higher rebate revenue. Moreover, the gross-to-net bubble (i.e., the dollar difference between sales at brand-name drugs’ list prices and their sales at net prices after rebates, discounts, and other reductions) has been growing at an exponential pace. The upward trend in the gross-to-net bubble reached $175 billion in 2019. Based on this trend and the fact that plan sponsors are not receiving full value of the rebates from PBMs, it is evident that rebates increase total drug spend of plan sponsors and only benefit PBMs.
The final and perhaps most long-term impact that rebates will have on plan sponsors is in the suppression of the biosimilar market. The greater use of less expensive biosimilars (essentially “generic” versions of biologic medications) has the potential to reduce overall drug spending. However, many health plans do not include biosimilars in their preferred tiers. This is because of the “rebate trap,” where PBMs prefer the higher cost, branded biologics that offer rebates, over cheaper biosimilar alternatives. The result is that when biosimilars do make their way to the market, many patients do not have access to them because their PBM does not cover it. These policies stifle advancements, and will, in the long term, keep plan sponsors beholden to higher cost, branded medications.
4.1.3 Harm to Providers
Finally, rebates also impact providers in several ways. First, PBMs preference of highly rebated drugs limits providers’ choice of optimal drug therapy for patients. Once again, this results in the PBM inserting itself in between the prescribers and their patients and violates the sanctity of the doctor-patient relationship. This is especially true with biosimilars. The greater use of biosimilars has the potential to reduce overall drug spending and provide greater clinical options for providers, including community oncology practices. However, due to rebates, many PBMs do not include biosimilars in their preferred tier, thereby preventing widespread adoption and cost savings.
In instances where biosimilars are included on formularies, this is done so inconsistently and on a patchwork basis, tied solely to the rebates that the PBM can extract from the drug manufacturer, and not the efficacy of the product. The result is that community oncology practices often are required to stock several different versions of very expensive biosimilars based on the rules of the patient’s PBM, rather than being able to prescribe and dispense the product that is best suited for their patients.
Rebates further intrude on the doctor-patient relationship when combined with step therapy, prior authorization, or other utilization management protocols. “Fail first” step therapy requires a patient to first fail once or twice on a medication specified by the PBM or health insurer before being allowed to “step up” to the therapy prescribed by the physician. In many cases, the medication dictated by the PBM or health insurer is not the least expensive medication, but rather, is the most profitable drug to the PBM due to rebates. The impact of step therapy, driven by rebating, is that it “takes the medical decision-making out of the hands of doctors” and puts it into the hands of the actuaries, accountants, and business people at the PBM, who are not choosing the drug that is most efficacious, or cheapest, or even most efficient – they are choosing the drug that is the most profitable.
4.2 What Does the Law Say?
Medicare Part D plan sponsors are required to submit DIR reports to CMS disclosing the total amount of rebates, inclusive of manufacturer rebates and pharmacy rebates, retained by PBMs regardless of whether such rebates were passed to Medicare Part D plan sponsors.
In the commercial market, many states have enacted laws that require transparency from PBMs and “pass through” pricing. For example, Delaware House Bill 194 enacted into law on July 17, 2019, permits the Insurance Commissioner to examine the affairs of PBMs, among other things. Likewise, under New York Senate Bill S1507A enacted into State Budget for the 2019-2020 Fiscal Year on April 12, 2019, PBMs are required to fully disclose to the Department of Health and plan sponsors the sources and amounts of all income, payments, and financial benefits. Similarly, Utah House Bill 272, which was enacted into law on March 30, 2020, requires PBMs to report all rebates and administrative fees to the Insurance Department including the “percentage of aggregate rebates” that PBMs retained under its agreement to provide pharmacy benefits management services to plan sponsors.
However, Maine Bill 1504, enacted into law on June 24, 2019, takes these reporting requirements a step further, and provides that “[a]ll compensation remitted by or on behalf of a pharmaceutical manufacturer, developer or labeler, directly or indirectly, to a carrier, or to a pharmacy benefits manager under contract with a carrier, related to its prescription drug benefits must be: A. Remitted directly to the covered person at the point of sale to reduce the out-of-pocket cost to the covered person associated with a particular prescription drug; or B. Remitted to, and retained by, the carrier. Compensation remitted to the carrier must be applied by the carrier in its plan design and in future plan years to offset the premium for covered persons.”
4.3 What Can Be Done?
If high drug prices meaningfully addressed then outsized negative impact of rebates, rebate aggregators, and the resulting high gross-to-net bubble must be addressed. Luckily there are several varied options available to the affected parties:
- Legislative
- Policymakers should enact laws that mandate PBMs and rebate aggregators to report drug manufacturer rebates procured by utilizing drugs dispensed to plan sponsors’ patients in a given year. Requirements set forth under 42 CFR § 423.514(d) are not sufficient to cast the light of full transparency on PBMs (and rebate aggregators) that contract with Medicare Part D plan sponsors.
- Laws should be enacted that allow plan sponsors to gain access to the drug manufacturer rebates reported by PBMs and rebate aggregators.
- Laws should be enacted that entitle Medicare Part D plan sponsors and state Medicaid agencies to conduct full and complete audits of PBMs and rebate aggregators and these entities should not have any ability to limit the scope and extent of such audits.
- Laws should be enacted that limit Medicare Part D plan sponsors’ financial obligation to CMS in the event that PBMs and rebate aggregators retained drug manufacturer rebates that were not relayed to Medicare Part D plan sponsors.
It should be called out that some in Congress have the mistaken belief that drug manufacturers are the primary beneficiary of rebates in terms of “buying” formulary access for their drugs. Although this may be true in a limited number of cases, the reality is that PBMs use rebates to extract – some would say “extort” – drug manufacturers to pay the rebate “toll” in order for PBMs to include these drugs on formulary or to avoid being part of a “fail first” step therapy scheme. Congress has been held hostage to PBMs and their corporate affiliated health insurers by threatening to increase plan premiums if rebates are eliminated or made illegal.
- Plan Sponsor Action
- As part of the PBM contracts, plan sponsors should:
- Require PBMs to seek approval from plan sponsors prior to delegating the rebate aggregation function to rebate aggregators.
- Require PBMs to disclose a list of rebate aggregators to plan sponsors.
- Require PBMs to disclose an unredacted contract with the rebate aggregator.
- Require PBMs to be pay fees to rebate aggregators for their services but such fees should not come from drug manufacturer rebates.
- Require PBMs to agree to rebate audits conducted by plan sponsors and/or third-party auditors at plan sponsors’ choosing.
- Require PBMs to report claims-level data on rebates collected on claims paid by pan sponsors.
- As part of the PBM contracts, plan sponsors should:
Pharmacy Direct and Indirect Remuneration Fees
As a result of a 2014 CMS rule change that went into effect in Plan Year 2016, PBMs have developed shrewd and calculated methods of financial engineering, maximizing their revenue at the expense of the patient, the Medicare Part D Program, and providers. This was accomplished through pharmacy direct and indirect remuneration fees, or “DIR fees.” DIR fees are typically post point-of-sale fees ranging from 1.5% to 11% of a drug’s list price assessed by PBMs upon network pharmacy providers, typically three to six months after the provider has dispensed the medication.
The concept of DIR fees arose out of Medicare Part D coverage for prescription drugs. Part D plan sponsors and Medicare Advantage plans offering drug coverage are paid by the government based on the actual cost for drug coverage. The actual cost is based on the Part D plan sponsor’s “negotiated price,” which is then used as the basis to determine plan, beneficiary, manufacturer (in the coverage gap), and government costs during the course of the payment year, subject to final reconciliation following the end of the coverage year.
Unfortunately, very few pharmacy price concessions have been included in the negotiated price at the point of sale. All pharmacy and other price concessions that are not included in the negotiated price must be reported to CMS as pharmacy DIR. As employers and plan sponsors are demanding a greater share of the PBM rebates, and as those rebates have been threatened with regulation by state and federal lawmakers, PBMs have gone “downstream” to make up for any rebate revenue shortfalls by assessing DIR fees on pharmacy providers. In fact, DIR fees categorized as pharmacy price concessions have increased 45,000 percent between 2010 and 2017, and have hit a whopping $9.1 billion in 2019.
Figure 6. Explosion of Pharmacy DIR from 2013 to Present

PBMs purport to pass a large portion of DIR fees to their plan sponsor clients, especially Part D plan sponsors – ironically, many of which are under the same corporation as the PBMs (e.g., CVS Caremark, one of the nation’s largest PBM, and SilverScript, the nation’s largest Medicare Part D plan sponsor, are both owned by CVS Health). However, no study has been conducted to match the deductions from pharmacy remittances for “DIR” with the DIR reported to CMS. Unfortunately, CMS cannot even perform such an audit today, as it does not require plans to submit DIR collected from each pharmacy, but rather requires DIR to be reported by drug, on an NDC number basis.
Even if pharmacy DIR fees are reported accurately, Medicare risk corridors allow a Part D plan sponsor that spends less than its bid estimate of costs to keep all savings up to 5% and a portion of those savings thereafter, which, in practice, allows PBMs and Part D plan sponsors to retain the vast majority of DIR fees collected. Thus, PBMs and Part D plan sponsors financially benefit from DIR fees.
Worse yet, DIR fees on expensive specialty drugs are typically calculated as a percentage of a drug’s list price. As such, DIR fees provide another incentive for PBMs to keep drug list prices high – high list prices yield not only larger rebates, but also larger DIR fees. As such, over the past several years DIR fees have become a larger percentage of the overall revenue that PBMs and Part D plan sponsors receive. Simply put, PBMs are making their money one way or another — rebates or DIR fees from pharmacy providers.
More problematic than the growth of DIR fees is the manner in which DIR fees are assessed on providers, especially community oncology practices. These fees are charged against community oncology practices based on their performance in a number of primary-care focused “quality metric” categories, which are totally unrelated and irrelevant to the cancer patients these practices treat. As a result, these community oncology practices have no meaningful ability to influence their performance scores – with no ability for upside – and such fees amount to nothing more than extortion from practices. Given the market clout of the top PBMs in terms of the percentage of prescription drugs they manage, community oncology practices simply have to pay these DIR fees to stay in network, lest they lose the ability to provide dispensing services to their patients.
These DIR fees are assessed after the point-of-sale. While they are sometimes recouped as soon as PBMs reimburse providers (i.e., extracted from initial reimbursements), in most cases DIR fees are assessed months after patients receive their medications. The total amount of DIR fees assessed on providers may not be known by providers until more than a year after a drug has been dispensed, as some PBM contracts create the potential for a partial or total refund of DIR fees (though a total refund is practically unobtainable).
DIR fees increase patients’ cost sharing responsibilities because patient out-of-pocket costs are based on an artificially inflated list drug prices at the point-of-sale; thus, in the case of Medicare patients, prematurely pushing them into the Medicare Part D “donut hole.” The cost of DIR fees also shifts the burden of drug costs to the federal government as more patients are prematurely pushed into the catastrophic phase of the Medicare benefit, resulting in higher financial contribution by the Medicare program. Ultimately, DIR fees weakens the overall benefit of the Medicare insurance benefit intended to provide health care coverage for our nation’s oldest and most vulnerable citizens.
Finally, DIR fees extracted from reimbursement to providers often results in drugs reimbursed below drug acquisition cost. Some speculate that this is yet another strategy by PBMs to ultimately drive pharmacy providers out of business so that the PBMs can take over the business with their retail, specialty, or mail-order pharmacies.
PBMs are able to effectively “extort” DIR fees due to their size and hegemony. As of 2018, three companies – UnitedHealth, Humana and CVS Health – covered over half of all Medicare Part D patients. Pharmacy providers do not have a meaningful choice but to accept the terms being provided to them – rejecting just one Part D plan could mean losing out on being able to service nearly a quarter of their Medicare Part D patients. PBMs know the power they hold and use it to its fullest extent.
5.1 Who is Impacted?
The expansion of DIR fees has had a substantial negative impact on both Medicare beneficiaries and the program as a whole. As confirmed in recent CMS studies, DIR fees ultimately shift financial liability from the Part D plan sponsor to the patient, then ultimately to the federal government, through Medicare’s catastrophic coverage phase. The shifting of financial liability away from the Part D plan sponsor and to Medicare and the patient is even more pronounced with specialty medications, such as oral cancer medications.
5.1.1 Harm to Patients
The primary harm to patients from DIR fees is that patients’ out-of-pocket costs are higher because they are based on list drug prices. Once again, PBMs have a vested financial interest to have drug list prices as high as possible as DIR fees are assessed as a percentage of the list prices for expensive specialty drugs. Medicare Part D patients find themselves paying more for their medications because they pay increased copayments and coinsurance on inflated point-of-sale list prices, which do not reflect the after-the-fact price adjustment in DIR fees that the PBM is clawing back from the pharmacy provider.
The use of DIR fees by PBMs has degraded the quality of the Medicare Part D benefit available for beneficiaries, all the while providing an additional lucrative revenue source for PBMs and affiliated Part D plan sponsors. It has shifted the benefit of the Medicare Part D program from those who rely on it for drugs, to those that do not use it, in the form of lower (or zero dollar) premiums. Meanwhile, DIR has put upward pressure on drug expenditures for those that use the benefit. Studies conducted by CMS have concluded that DIR fees increase out-of-pocket costs for Medicare patients at the point of sale.
Consider for example, that Medicare Part D beneficiaries’ cost sharing is based on the PBM-determined rate at the point-of-sale. DIR fees are by definition not assessed at the point of sale. Thus, the patient’s copayment or coinsurance that is based on the price at the point-of-sale is artificially inflated. CMS similarly concluded that DIR fees cost patients money, noting “[w]hen pharmacy price concessions and other price concessions are not reflected in the negotiated price at the point of sale (that is, are applied instead as [Direct and Indirect Remuneration] at the end of the coverage year), beneficiary cost-sharing increases.”
Likewise, up until the end of the 2020 plan year when the “donut hole” existed in the Medicare Part D Program, DIR fee programs pushed patients through the coverage stages much faster. Within the donut hole, patients pay 25% of the drug cost based on the (inflated) list price at the point-of-sale. The concern that patients continue to foot the bill for increased costs is not hidden from scrutiny as a group of 21 U.S. Senators urged HHS to address DIR fees because “beneficiaries face high-cost sharing for drugs and are accelerated into the coverage gap (or “donut hole”) phase of their benefit.”
In addition, despite PBMs’ purported justifications for such programs, DIR fees have not benefitted the quality of Part D plans offered to Medicare beneficiaries. For example, SilverScript had a 4.0 Star Rating from Medicare in 2018 (based on 2017 data), but saw its score drop to a 3.5 Star Rating in 2019 despite the widespread usage of DIR fees. At the same time, as the impact of DIR fees has increased dramatically since 2016, patients have also been impacted by diminished access to care as providers facing decreased net reimbursement are forced out of business, forcing patients to receive services from pharmacies owned by or affiliated with the very PBMs and Part D plan sponsors extracting DIR fees (see, Section 6, infra).
5.1.2 Harm to Plan Sponsors
Just as DIR fees negatively impact patients, PBM-Imposed DIR fees shift costs away from Part D plan sponsors, while increasing the costs to the Medicare program (and in turn, the taxpayer) for catastrophic coverage and subsidy payments. As mentioned, when a Medicare beneficiary is pushed through the benefits tiers and reaches the “catastrophic coverage” stage, the cost of services shifts to 80% paid by Medicare, while only 15% paid by the plan sponsors. The government covers these costs in part by turning to the reinsurance marketplace. From 2007 through 2018, a period similar to when CMS saw DIR fees from pharmacy price concessions increase by more than 45,000 percent, reinsurance costs of Medicare soared by 411%. Part D plan sponsors and their PBMs have a financial incentive to move Medicare beneficiaries into the catastrophic phase of coverage, to the detriment of the taxpayer.
In fact, the National Community Pharmacists Association (NCPA) commissioned a report by Wakely Consulting Group, LLC to estimate the cost savings that would occur if congress prohibited retroactive reductions in payments by Part D plan sponsors in the form of DIR fees. Wakely Consulting Group, LLC found $3.4 billion in Part D payments over a nine-year period if these fees were prohibited.
Unfortunately, the harm from DIR fees goes beyond the Medicare program and American taxpayers. Like rebates, DIR fees have the effect of driving up the cost of drugs, through higher list prices. From 2013 to 2019, DIR fees rose from $229 million to an estimated $9.1 billion. Most striking, however, is that DIR fees now account for more than 18% of all Medicare rebates received by Part D plans. This increased reliance on DIR fees relative to drug rebates, both of which are tied to the list price of drugs, highlights the upward pressure DIR fees have placed on list prices for drugs. During this same period, drug list prices grew between 10-15% per year. Meanwhile, net prices have been relatively flat throughout this time period. These inflated list prices are felt by all plan sponsors – especially employers and state Medicaid programs – who do not receive any of the supposed benefits of DIR fees (such as lowered premiums).
PBMs have used their consolidation in the marketplace to use DIR fees and rebates in concert, fueling higher drug prices, while adversely impacting cancer care.
5.1.3 Harm to Providers
To say that DIR fees have had an adverse impact on providers is an understatement. DIR fees decrease pricing transparency creating uncertainty as to the true real reimbursement rates for drugs, very often driving reimbursement rates below the providers’ acquisition cost of drugs (see, Section 8, infra).
The metrics utilized by PBMs in implementing DIR fee programs are typically completely inapplicable to community oncology practices. Specifically, community oncology practices dispense primarily (and almost exclusively) specialty medications for cancer patients. As such, they have virtually no ability to influence their performance based on PBMs’ “quality metric” categories measuring patient drug adherence relating to cholesterol, heart disease, and diabetes medications, which are relevant to dispensing general medications, not specialty drugs.
Worse yet, adherence-based metrics are particularly problematic and in cases not only wholly inapplicable in treating cancer patients, but also may be very dangerous. Community oncologists are extremely vigilant about monitoring their patients’ cancer medication regimens and may temporarily discontinue or “hold” medications until a patient’s status returns to an acceptable level, especially relating to adverse drug side effects. The period during which the medication is “held,” or therapy is temporarily discontinued, is wrongly and obtusely measured by the PBM as a lack of adherence in one of the few areas where the community oncology practices may be measured, ultimately causing the community oncology practices’ performance to decrease, and the DIR fee assessment to subsequently increase.
Consider, for example, Imbruvica (ibrutinib), which is dispensed by many community oncology practices to treat mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL). Studies have shown that Imbruvica tends to cause hematologic effects such as neutropenia and thrombocytopenia in MCL and CLL. If these adverse events occur at certain levels, the standard of care – as articulated directly by the FDA-approved package insert – is to hold the medication until the patient’s lab values return to normal ranges. This can happen in as many as 46% of cases, resulting in discontinuing the patient’s medication for up to a month. If community oncology practices are required to continue to dispense this drug, it will result in additional (and avoidable) costs to Medicare for the discontinued fills, as well as potential harm to the patient (along with potentially increased costs to Medicare for associated medical costs).
Further, due to the high cost of specialty drugs, and in particular, oncology medications, any small change in perceived adherence rates due to the purposeful physician-directed temporary discontinuation of therapy results in unreasonably low reimbursement rates. Many PBMs justify their DIR fee programs as being designed to influence providers to deliver better care to patients in their Medicare Part D networks. On that clinical basis, if community oncology practices were to be “influenced” by the PBMs’ DIR fee metrics by adhering to a medication when the FDA-approved label calls for the therapy to be held, patients would suffer. As such, community oncology practices are often left without any meaningful way to impact PBMs’ so-called “quality metrics” and improve their DIR fee performance.
Ultimately, community oncology practices have no way out. For them, due to the clout and market leverage of PBMs, DIR fees are simply a form of extortion that community oncology practices are forced to pay.
5.2 What Does the Law Say?
The most directly applicable legal principles relating to pharmacy DIR fees are found in the federal Any Willing Provider law. Within the federal Any Willing Provider law, CMS expressly recognized that unreasonably low reimbursement, which often result after accounting for DIR fees, violates the federal Any Willing Provider law. As it relates to the methodologies being used to assess DIR fees, performance criteria, and the manner in which PBMs and Part D plan sponsors are using those programs must also be reasonable and relevant. For community oncology practices, performance criteria that they are unable to influence or performance criteria that does not reasonably measure optimal cancer care can run afoul of the federal Any Willing Provider law.
In addition to explicit statutory language and CMS guidance, many of these principles are incorporated within, and apply directly to, the contract between PBMs and community oncology practices. PBM contracts include explicit obligations that the PBMs will comply with federal code, statues, rules, and CMS guidance, including but not limited to the Medicare Part D Provider Manual. These contractual obligations are not included in the contract with pharmacies by choice, but rather federal law requires these terms to be included in the contract between CMS and plan sponsors, and in contracts with their first tier entities (including PBMs, and in contracts between PBMs and pharmacy providers). This creates affirmative obligations on PBMs to comply with these laws, as well as the ability for pharmacy providers to directly challenge PBMs for breaches of contract when PBM actions do not comply with federal law.
In January 2022, CMS introduced a proposed Final Rule that would alter the way PBMs and Part D plan sponsors are required to report DIR fees. In particular, CMS has proposed that PBMs and Part D plan sponsors report the lowest possible reimbursement to pharmacy providers (inclusive of all potential DIR fees) as the “negotiated price.” While this proposed rule (if finalized) could have the result of removing the financial incentive for PBMs and Part D plan sponsors to institute retrospective DIR fees, it does little to protect pharmacy providers against unreasonably low reimbursement rates or wholly irrelevant “quality” metrics when assessing DIR fees.
5.3 What Can Be Done?
- Legislative Solutions
-
- Federal legislation should be enacted requiring that any DIR fee program (i) be tied to relevant quality programs to the specialty being measured; (ii) actually measured on an individual pharmacy level; (iii) provide equal opportunity for upside performance (i.e., not just a way for PBMs to “rig” the program to always measure downside performance resulting in DIR fees extracted from the provider); and (iv) require that DIR fees be applied equally and fairly across all network pharmacies, specifically including PBM-owned or affiliated pharmacies).
- Federal legislation should require that all pharmacy price concessions, including DIR fees, be included in the negotiated price at point-of-sale.
- Federal legislation should give CMS greater latitude in regulating the reimbursement structure between Part D plan sponsors and pharmacy providers.
- Regulatory
-
- CMS should issue regulation providing “guard rails” on what constitutes reasonable and relevant terms and conditions, and clarify that whether given terms are “reasonable” or “relevant” can be adjudicated in a private contractual dispute between Part D plan sponsors/PBMs and pharmacies.
- CMS should initiate complaints against Part D plan sponsors and PBMs who have failed to pass on negotiated prices to patients at the point-of-sale, when DIR fees were known or knowable (i.e., the PBM maintained a minimum range of DIR fees that were to be assessed against every pharmacy no matter what).
- CMS should initiate complaints against Part D plan sponsors and PBMs who have not paid providers based on reasonable and relevant terms and conditions, including through unreasonably low reimbursements, or irrelevant performance criteria.
- CMS should require reporting of pharmacy DIR fees by both NDC number and pharmacy National Provider Identifier (NPI) allowing for full end-to-end audits of the flow of money from pharmacies to the Medicare program. The results of these audits should be made available to the public.
Restrictive Networks, Credentialing Abuses, and Artificial Barriers of Entry
PBMs maintain a monopoly-like grasp on the industry, the natural result of which is the inability of patients to freely choose a provider based on his or her personal health care decisions, as opposed to the mandates of his or her PBM. As noted previously, only three PBMs process more than three-quarters of all prescription claims: CVS Health, Express Scripts, and OptumRx, while five PBMs process over 80% of all prescription claims. Each of the three major PBMs share common ownership with a major insurer and in turn with a mail-order and/or specialty pharmacy. These vertical, integrated relationships allow the PBMs to control the pharmaceutical supply chain, and erect superficial barriers to entry or even outright exclude entire classes of potential pharmacy providers.
This is particularly pronounced in the context of cancer care, where the introduction of new oncology therapies over the past several years, specifically, oral treatments for cancer and related conditions, presents new challenges for patients, plan sponsors, and providers alike. Between 2017 and 2019, there have been over twenty-four new oral cancer medications introduced into the marketplace. In 2020 alone, ten new oral oncolytics were approved by the FDA. As it stands, oral oncolytics make up 25% to 35% of cancer medications in development, making it likely that over the next several years, oral therapies will encompass an indispensable component of any treatment plan for cancer patients. While traditional chemotherapy infusion therapy that is “administered” is covered under a patient’s “medical” benefits, oral oncolytics that are “dispensed” are being shifted to the patient’s “pharmacy” benefits, managed by PBMs. Unlike chemotherapy administered in the clinic setting, the advent of oral oncolytics have given the PBMs a tremendous new opportunity to control cancer care and divert prescriptions and profits to themselves.
These new oral cancer medications can be extremely expensive, often ranging more than $10,000 per month. This is what is attracting PBMs, and as a result, PBMs have attempted to use their market size and leverage to limit dispensing of oral oncolytics through certain specialty and/or mail-order pharmacies, most often their own or affiliated pharmacy.
PBMs use several different tactics to maintain their control over where patients receive their care. The first and foremost of these is creating restricted networks, blocking access to any provider that is not affiliated with their PBM. In these instances, the PBM will contend that the network is “closed” or that there is no “network,” and thus, pharmacy providers are not even given the opportunity to apply for network admission. This occurs more frequently in the commercial insurance space involving employer-sponsored plans, but can also involve Medicaid managed care programs, where the PBM will require patients to receive their cancer medication from the PBM’s wholly-owned or affiliated pharmacy, and no one else. This is anticompetitive conduct – pure and simple – where patients are trapped into using one particular provider not based on the quality of care provided by that provider but based on the financial arrangements and the corporate affiliation between the pharmacy provider and the PBM and/or health insurer.
A related, but slight variation of this tactic is to restrict access to certain classes of providers (i.e., retail pharmacies), while excluding wholesale other classes of providers (i.e., dispensing physician practices). For example, beginning in early 2016, CVS Caremark espoused a self-serving stance that dispensing physician practices were now to be deemed “out-of-network” and no longer able to participate in Medicare Part D networks. This would have the effect of dramatically interrupting the ongoing relationship between treating oncologists and their patients. CVS Caremark later backtracked on this position and began allowing “grandfathered” dispensing physicians (i.e., those that previously held a contract with the PBM) to continue in-network, but delayed the processing of any new, non-grandfathered dispensing physician practices. In another instance, in January of 2018, Prime Therapeutics (Prime) – the PBM owned by a consortium of approximately twenty-two Blue Cross Blue Shield plans – announced that it would no longer accept any new dispensing physicians into its pharmacy networks on the alleged basis of “fraud, waste, and abuse” concerns and a commitment to maintaining to compliant networks. Without providing any further details, Prime claimed that Dispensing Physicians did not adhere to Prime’s Provider Manual. This trend expanded to existing in-network dispensing physicians actively servicing patients when, recently, Prime announced that it would also terminate existing, or “grandfathered” dispensing physicians from its networks. Despite having credentialed, contracted, and paid dispensing physicians as “in-network” Medicare Part D providers for over a decade, Prime seemingly unilaterally took the position that dispensing physicians are now considered “out-of-network providers” under Medicare Part D. Like wholesale network exclusion, these practices disadvantage vital providers while allowing PBM-owned or affiliated pharmacies to capture a greater share of prescription volume.
Even in instances where a PBM nominally allows a community oncology practice to apply for network participation, the PBM can still place other barriers in the way of providers being able to service their patients by imposing onerous credentialing processes. For a community oncology practice to service patients within a PBM’s network, PBMs require that the provider adhere to specific and extremely onerous, credentialing requirements, including the requirement that the provider maintain certain accreditations. These conditions are made even more onerous where PBMs delay the review of credentialing applications (seemingly with the intention to avoid admitting these providers), enact credentialing applications with terms and conditions designed to keep out providers (rather than ensuring the quality of providers) or allow participation but at rates so low that reimbursement may not even cover the acquisition cost of a drug. These obstructionist policies harm patients, degrade the quality of prescribers and benefit only PBMs that are incentivized to continue to these illegitimate practices.
Finally, even when a community oncology practice has ultimately been admitted into a PBM’s network, PBMs continue to utilize other tactics to drive patients away from community oncology practices, and towards PBM-owned or affiliated pharmacies. This includes tactics such as patient slamming and claim hijacking (see, Section 7, infra), misleading communications aimed at steering patients to PBM-owned or affiliated pharmacies, and creating patient incentives for patients (such as lower copays, larger days’ supply or free products/services) to utilize preferred PBM-owned or affiliated pharmacies. PBMs also utilize other tactics, such as abusive auditing practices (i.e., requiring the production of thousands of pages of documentation to support claims billed) and terminating providers without cause or on pretextual bases (i.e., that they only dispense one class of medications).
PBMs employ these tactics to maintain their oppressive market dominance. But at the same time, in a vicious cycle, these tactics are themselves the consequence of the horizontal and vertical consolidation within and between insurance and PBM markets, which has created merged entities with such oppressive power that it a virtual chokehold on community oncology practices and pharmacy providers. The result of these tactics is that patients are steered away from receiving care at their community oncology practices, and forced to receive care from PBM-owned or affiliated pharmacies. This is not only without regard to the impact on patient care and outcomes, but as the chart below demonstrates, only continues to prop up higher drug prices and charges.

6.1 Who is Impacted?
The overall lack of industry standards and oversight in the PBM credentialing sphere has led to arbitrary denials and lengthy, costly application processes, that ultimately have a negative impact on a community oncology practice’s ability to focus on patient care. Instead of allowing community oncology practices to enter into their networks, PBMs attempt to limit the dispensing of oral oncolytics through their own specialty pharmacies, leading to poor patient compliance and adherence to life-saving treatments, causing the quality of cancer care to suffer.
These tactics have had negative impact all across the spectrum, affecting patients, health care payers (including Medicare, Medicaid, employers and taxpayers), and providers.
6.1.1 Harm to Patients
These exclusionary practices – whether they be unreasonable barriers to entry or outright exclusion of certain classes of providers – result in serious harm to patients, specifically those who are seeking the services of community oncology practices that have been excluded from a PBM specialty network. For one, these exclusionary practices destroy existing patient-provider relationships. In early 2016, when CVS Caremark undertook re-interpreting longstanding CMS regulations, it did so in such a way as to effectively cut out physicians from continuing to dispense medications to their existing Medicare Part D patients. PBMs have no regard for the continuity of these vital health care relationships and their impact on patients’ well-being and outcomes.
This is critical, as patients are more likely to raise certain questions or concerns about their medications, when these medications are dispensed by community oncology practices. To strip patients, who are facing serious life-threatening diseases, of that important patient-provider relationship could result in serious patient harm. This also has the effect of decreasing medication adherence, which would further affect patients, especially those undergoing life-saving treatments at community oncology practices.
The ultimate outcome of creating restricted networks or excluding entire classes of providers, namely, that patients are essentially required to obtain medications at a PBM-owned or affiliated pharmacy. It is well-documented that when the PBM-owned or affiliated pharmacy is responsible for filling the patients’ prescriptions, it results in worse care. The near-monopolistic control of the network, combined with the lack of patient choice, remove any checks and balances on the quality of the care being provided.
Consider, for example, a patient battling cancer was denied life-saving medications by a PBM due to the PBM being unwilling to enter medications into its computer system. In another example, a patient had been diagnosed with Philadelphia chromosome-positive + chronic myeloid leukemia and had been responding positively to “180mg” of a certain medication. However, according to the patient’s PBM, the medication had to come from the PBM’s mandated mail order specialty pharmacy instead of a pharmacy of their choice. Since the medication was not available in a single 180mg dosage form, the prescription clearly indicated that the patient was to receive a “100 mg tablet and an 80 mg tablet.” Instead, over the course of the next several months, the PBM pharmacy dispensed either a 100 mg tablet or an 80 mg tablet, but never both. Ultimately, the patient did not respond well to the lowered dosages of the medication. Finally, in a particularly disturbing example, a colorectal cancer patient was prescribed a common oral medication that had been on the market for nearly twenty years. The patient’s PBM mandated that the patient fill the prescription at a large, well-known specialty pharmacy, and the patient’s oncologist prescribed the medication to be taken in rounds with the following specific instructions: ‘two weeks on, one week off.’ The PBM mail-order pharmacy neglected to include the ‘one week off’ instruction on the label, and as a result, the patient ended up in the intensive care unit of a hospital.
Unfortunately, patients often do not have any ability or choice to switch their PBMs in order to have control over which pharmacy provider from whom they would like to receive service. PBMs who undertake these restrictive practices are typically selected by the patient’s employer (or sometimes by the insurance company selected by the patient’s employer). The patients are two, sometimes three steps removed from any part of the decision-making process. Since most patient get their health care coverage through their jobs, the only way a patient can exert any control over the network of pharmacy providers is to change jobs and hope that their new employer utilizes a different PBM’s network. But, in a world where three PBMs account for nearly 80% of the marketplace, the odds of getting a better PBM are slim to none.
The PBMs know the level of power that they wield. And their focus is on profits, not patients. Ultimately, given the acute focus on patient care inherent in community oncology practices, patients suffer when those providers are forced out of the space.
6.1.2 Harm to Plan Sponsors
In addition to patients, these exclusionary practices harm plan sponsors, such as Medicare and Medicaid, because they cause an artificial rise in the cost of specialty medication, particularly within the oncology space. Specifically, the exclusion of community oncology practices from PBM networks require more patients to utilize PBM-owned or affiliated mail-order and/or specialty pharmacies. This, in turn, leads to exponentially more waste of medication, causing increased costs to plan sponsors. Mail-order pharmacies, without proper access to patient outcomes, routinely dispense 90-day supplies of medications. In several instances, patients continue to receive medications despite their repeated requests to have the mail-order pharmacy cease sending medication, often due to a change in their course of treatment. In more tragic cases, the PBM mail-order pharmacies continue to dispense medications to the patient’s residence despite the patient having passed away, leading to the waste of unwanted, expensive medications.
Moreover, when pharmacy care is diverted from community oncology practices to PBM-owned or affiliated pharmacies, plan sponsors lose out on tremendous value-based contracting opportunities. In the Medicare space, CMS is developing new payment and delivery models designed to improve the effectiveness and efficiency of specialty care. Among those specialty models is the Oncology Care Model, which aims to provide higher quality, more highly coordinated oncology care at the same or lower cost to Medicare. The Oncology Care Model “provides an incentive to participating physician practices to comprehensively and appropriately address the complex care needs of the beneficiary population receiving chemotherapy treatment and heighten the focus on furnishing services that specifically improve the patient experience or health outcomes.” PBM exclusionary practices would thwart this initiative. Likewise, in the private sector, value-based care (VBC) innovations are on the rise, increasing the quality while lowing the overall cost to health care payer and their patients. The ability to tie benefits to providers and value to patients is critical to aligning interests in the health care space and has long been a long-term goal of health policy experts. However, this type of integration of medical and pharmacy care is against the interest of current PBM practices to implement. Absent changes to PBM regulation, the federal government will be unable to achieve some of the same cost-saving/quality improving measures as is being utilized in primarily the self-funded employer sponsor health care space.
Unfortunately, these lost opportunities are not made up for in savings garnered by PBMs, and in fact, quite the opposite has occurred. As illustrated in the figure on page 36, the exclusion of community oncology practices and other independent providers allows PBMs to pocket more through their wholly-owned or affiliated mail-order and specialty pharmacies.
In a study conducted by Ohio’s Medicaid Managed Care Pharmacy Services, PBMs billed taxpayers 8.8% more for medications than what they paid pharmacies. This difference, commonly referred to as “spread” has been growing and is typically the highest on specialty medications, such as oral oncolytics. Worse yet, similar data has shown that the spread between plan sponsor funded PBM revenue and pharmacy-captured reimbursement has increased over time. In short, PBMs are keeping more and more revenue from health care costs to the detriment of others in the health care space.

Ultimately, when compared to costs of PBM exclusionary practices, the savings associated with dispensing by community oncology practices are palpable. Reports estimate that physician point-of-care dispensing could save seniors and taxpayers over $20 billion in Medicare Part D alone.
6.1.3 Harm to Providers
An increasingly important component of the physician-patient relationship with oncology is the dispensing of medications to patients through the community oncology practice, at the site of care. Excluding community oncology practices from PBM networks prevents physicians from providing consistent care to their patients.
When PBMs impose unreasonably high or arbitrary requirements for network admission, designed for no purpose other than to serve as an artificial barrier of entry, they place immense and undue burdens on community oncology practices seeking to service their patients. As noted above, these credentialing standards often require a provider to hold multiple forms of accreditation, such as URAC and ACHC. These specified accreditations are often not the most relevant or appropriate form of accreditation for community oncology practices, and do not constitute the most applicable form of endorsement based on the unique and specialized services provided by community oncology practices.
Between the standards set forth under the Oncology Care Model (OCM) and Quality Oncology Practice Initiative (QOPI®) Certification Program, community oncology practices also attain high standards of practices, validated by third parties, that obviate the need for separate accreditation. For example, QOPI has a certification program specifically designed for clinical oncology practices as this process “can routinely evaluate practice performance against quality measures and standards established by experts in the oncology field.” Likewise, through the CMS-created OCM, community oncology practices have entered into payment arrangements that include financial and performance accountability for episodes of care surrounding chemotherapy administration to cancer patients. The practices participating in OCM have committed to providing enhanced services to Medicare beneficiaries such as care coordination, navigation, and national treatment guidelines for care. The fact that CMS has involved itself in the creation of this type of model with standards that directly correlate to community oncology providers demonstrates that these two programs (OCM and QOPI) would be the best industry standards to judge a network provider. Moreover, requiring dual accreditation – including URAC accreditation in Specialty Pharmacy – apart from being redundant, also increases the risks that the provider will have multiple, sometimes contradictory compliance requirements, needing to comply with not just ACHC standards, but also URAC standards, which at times can be diverging. Finally, these accreditations can be prohibitively expensive and costly, making it impracticable for providers to undertake the steps necessary to even seek admission to the networks.
Likewise, when PBMs take steps to delay credentialing, this too harms pharmacy providers. Community oncology practices have to divert considerable amount of time and resources to respond to repeated follow ups on their credentialing applications under normal circumstances. However, when a PBM “slow rolls” an application and takes months to review and respond to inquiries, this has often led to the PBM asking the provider to provide the same documentation over, and over and over again (i.e., licenses that expire and are renewed over the course of the sometimes 18-month long credentialing process). This takes time away from being able to service patients.
But perhaps the most direct way providers are harmed by these tactics is through the actual effects of network exclusion. Due to the size and market share of each PBM (see, Section 3, supra), a PBM termination or exclusion often spells irreparable harm for a provider seeking to participate in pharmacy networks and/or the Medicare Part D program. Particularly alarming is the fact that about two-thirds of all Medicare Part D Prescription Drug Plan enrollees are concentrated in networks across just three payers: OptumRx, CVS Caremark, and Humana. Exclusion from any one of these payers could make dispensing simply not a viable option for a community oncology practice.
6.2 What Does the Law Say?
Among all the barriers that PBMs put in front of providers – including onerous credentialing processes, restricting network access, steering to owned or affiliated pharmacies – the core legal principles largely tie back to rules promulgated around freedom of patient choice and network participation. Remarkably, there are several federal and state laws on the books that seek to safeguard the rights of patients to select the provider of their choice, or to protect community oncology practices from undue network termination or exclusion. In the federal statutes establishing and governing the Medicare program, Congress has included explicit “Any Willing Provider” requirements, which relate directly to network access for Medicare providers, including community oncology practices. These statutes apply to all Part D plan sponsors, as Part D plan sponsors are under the purview of CMS, pursuant to contracts between the Part D plan sponsors and CMS.
The Medicare Any Willing Provider law (42 U.S.C. § 1395w-104) explicitly requires that all Part D prescription drug plans permit “the participation of any pharmacy that meets the terms and conditions under the plan.” The federal “Any Willing Provider” law further prohibits health insurers from creating exclusive provider networks – or unduly barring entry to such networks (such as through artificial barriers of entry) – to which insured patients are directed to the exclusion and detriment of non-network providers. In fact, as it relates to credentialing abuses, CMS has also questioned whether mandatory accreditations should be considered “standard terms and conditions” of a network, and whether PBMs should instead explore other reasonable and relevant alternatives to ensure quality assurance and actual improved patient care, particularly where certain accreditation requires may be arbitrary and not directly proven to ensure quality assurance.
Likewise, federal law provides protection directly for patients to have the freedom to select a provider of their choice. Pursuant to 42 C.F.R. § 431.51(a), Medicaid beneficiaries may obtain services from any qualified Medicaid provider that undertakes to provide services to them. However, plan sponsors commonly use preferred networks to incentivize beneficiaries to fill claims at pharmacies of the Plan’s choice (rather than the beneficiary’s choice), by offering reduced co-pays at preferred pharmacies.
Several states also maintain their own versions of “Any Willing Provider” protections. For example, North Carolina’s Any Willing Provider Law provides that a health benefit plan shall not “[p]rohibit or limit a resident of th[e] State … from selecting a pharmacy of his or her choice when the pharmacy has agreed to participate in the health benefit plan according to the terms offered by the insurer,” or “[d]eny a pharmacy the opportunity to participate as a contract provider under a health benefit plan if the pharmacy agrees to provide pharmacy services that meet the terms and requirements, including terms of reimbursement, of the insurer under a health benefit plan…”
Similarly, Tennessee’s Any Willing Provider Law provides similar limitations on the ability to exclude providers such as community oncology practices, mandating that “[n]o health insurance insurer and no managed health insurance insurer may… deny any licensed pharmacy or licensed pharmacist the part to participate as a participating provider in any policy, contract, or plan on the same terms and conditions are offered to any other provider of pharmacy services under the policy, contract or plan” or “[p]revent any person who is a party to or a beneficiary of any policy, contract, or plan from selecting a licensed pharmacy of the person’s choice … provided that the pharmacy is a participating provider under the same terms and conditions of the contract, policy or plan as those offered any other provider of pharmacy services.”
These laws prohibit not just outright network exclusion, but also a host of other PBM practices aimed at requiring that patient use their wholly-owned or affiliated pharmacies.
At both the federal and state levels, policy recognizes the importance of provider access and, ultimately, competition via the enactment of these “Any Willing Provider” rules. Unfortunately, these laws have not been without attack by the powerful PBMs, and in few instances do they provide pharmacies a private right of action to enforce and ensure they are meaningfully applied.
6.3 What Can Be Done?
- Legislative
-
- Congress should enact federal legislation that provides a private right of action for community oncology practices to exercise their rights under the federal Any Willing Provider law, particularly when they are unfairly excluded from PBM networks and a private right of action will allow the enforcement of a regulation by a private party, such as a community oncology practice, allowing for litigation or the threat of litigation to incentivize compliance of the law.
- Congress should enact state legislation that curbs credentialing abuses and provides for stronger Any Willing Provider laws and provides for a private right of action for community oncology practices to exercise.
- Regulatory
-
- CMS should pursue complaints against PBMs for their construct of artificial barriers of entry and failure to adhere to the establishment of reasonable and relevant terms and conditions of participation.
- CMS should also enact regulation to specify “reasonable” and “relevant” standards of participation to allow for defined requirements PBMs must adhere to.
- CMS should issue regulation providing “guard rails” on what constitutes reasonable and relevant terms and conditions, and clarify that whether given terms are “reasonable” or “relevant” can be adjudicated in a private contractual dispute between Part D plan sponsors/PBMs and pharmacies.
- State Departments of Insurance should pursue complaints against PBMs for violations of Any Willing Provider Laws, and Medicaid Free-Choice-of-Provider provisions.
- Plan Sponsor Action
-
- Plan sponsors should require PBMs to seek approval from plan sponsors prior to establishing a standard and/or qualification for a provider network.
- Plan sponsor should have the full and final authority to make any modification to a standard and/or qualification for a provider network.
- Plan sponsors should retain the right to participate in an administrative hearing requested by a provider who has been terminated or rejected from a PBM’s provider network.
- Plan sponsors should retain the full and final authority to make accept or deny a provider’s request to participate in a PBM’s provider network.
Prescription Trolling, Patient Slamming, and Claim Hijacking
A patient’s decision on where to fill his or her medication, especially a cancer medication, is of immense importance. Cancer patients require ease of treatment and as little confusion as possible, in order to have a positive outcome. Based on these principles, Section 30.2.2.3 of the Medicare Prescription Drug Benefit Manual prohibits PBMs and Part D plan sponsors from “Steering of physicians or beneficiaries to a sponsor’s and/or PBM’s own mail order Pharmacy.” Such prohibition specifically includes steering of prescribers’ patients to a specialty pharmacy owned by or affiliated with a plan sponsor/PBM and most PBM contracts require adherence to CMS Guidance and contain compliance with law provisions.
Despite the law, there are innumerable instances where the PBMs haves effectively utilized claims or fill data and sought to move the prescription away from the provider of the patient’s choice and toward the PBM’s wholly-owned or affiliated pharmacy. This practice, sometimes referred to as “prescription trolling,” “patient slamming,” or “claim hijacking,” plays out fairly consistently. A typical case might involve a situation where the PBM allows the provider to submit a claim (typically a high-cost specialty medication), then reject it claiming that it required a prior authorization (PA). Then, once the provider has done all the required work to obtain the approval for the PA, it is subsequently rejected once again by the PBM, this time for the apparent reason that it “must” be filled at the PBM-owned or affiliated specialty pharmacy.
Pharmacy providers typically transmit prescription claims (and sometimes PA requests) to the patients’ PBM for purposes of having it adjudicated and receiving reimbursement. Such transmissions clearly contain protected health information (PHI) and are directed solely at the PBM acting as the claims adjudicator. Instead of simply reviewing and processing this claim, in its fiduciary capacity as the PBM, the PBM improperly and unlawfully accesses the PHI, and illegally communicates the claim information to its related entity (a PBM-owned specialty pharmacy). While the PBM is processing the PA, the PBM-owned or affiliated pharmacy surreptitiously communicates to the patient, prescriber, or both, with the goal of having the prescription filled at the PBM-owned or affiliated specialty pharmacy. Community oncology practices have documented some egregious instances where the PBM blatantly lied to the patient and pharmacy staff, saying the prescribing physician had authorized the transfer, when in fact, they clearly had not. Further, with complete disregard to not only patient privacy laws, but also state Pharmacy Practice Acts, PBM-owned specialty pharmacies have brazenly filled and dispensed the medication in complete absence of having an actual, signed prescription in hand.
Worrisomely, more deceitful and underhanded variations of this also exist. In some instances, PBM-owned or affiliated pharmacies have sought to mislead patients into thinking that their physician wants the prescription to be filled at the PBM-owned or affiliated pharmacy, or otherwise imbed prescription transfer documentation in the information the PBM provides to the physician in order to renew the prescription for refill (and the physician unknowingly signs to have the prescription transferred).
7.1 Who is Impacted?
7.1.1 Harm to Patients
A direct result of prescription trolling is severe confusion and distress for cancer patients, who are caught in the middle, uncertain of when or from where they will receive their next dose of their life saving medication. These concerns in the context of prescription trolling go beyond those when a PBM takes steps to create a restricted network (see, Section 6, supra); it is far more insidious here. While patients cannot be compelled to fill their prescription from a specific dispenser, many report receiving correspondence from their PBM implying that they must use a pharmacy owned by or affiliated with the PBM. These letters often explain that the insurance company has its own “preferred” pharmacy, from which the patient may already be receiving other prescribed drugs and offer for the patient to also get their oral cancer drug from this same source. PBMs may try to entice patients to select their “preferred” pharmacy through lower patient copayments to the patient only for the patient to later realize their oral oncolytics cost more at the “preferred” pharmacy than a non-preferred provider. Many patients find this confusing and do not understand the repercussions that jeopardize the monitoring, care control, and clinical management that they receive at their community oncology pharmacy, and they mistakenly, or unintentionally, switch their drug dispenser.
Many patients may require special assistance from their community oncology practice that has documented and understands their medical history, monitors for drug interactions between their medications, and is able to make appropriate dosing adjustments at the time of administration. Furthermore, a patient who is switched over to a PBM-owned or affiliated mail-order pharmacy often has his/her medication shipped from a distance (sometimes several states away), running the risk that the drug could be rendered ineffective in treating that patient’s condition due to a lack of sufficient temperature control during transit. In short, the harm can literally be deadly for patients with cancer, because of the disease and drugs involved – medications arriving too late or failure to timely amend dosing regimens can be the difference for life and death for these patients.
Perhaps worst of all, PBMs and their wholly-owned or affiliated specialty pharmacies have been known to employ underhanded tactics to “hijack” the prescription. In one particularly egregious instance, a PBM-affiliated specialty pharmacy contacted a community oncology practice claiming that one of the clinic’s patients had requested that his lung cancer medication be transferred to the PBM-affiliated pharmacy and demanded the clinic’s immediate compliance in the matter. Surprised by the news, the oncologist contacted the patient to inquire about his decision, only to discover that this was the first time the patient had heard of the matter. “Please do not transfer it anywhere else!” the patient requested. “I want to get it filled through the dispensary. I did not ask for this. I love being able to get this right away and with no hassles. I was on an oral chemo before and it was filled by a specialty pharmacy and I always was getting it late, missed a few days of medication sometimes and had numerous phone calls from them. They never seemed to know what was going on with my medication.” As evidenced by this true story account, patients receiving their oral drugs from a community oncology practice have access to those drugs within 24 hours of prescribing, and they can begin treatment immediately. Patients receiving their oral cancer drugs through a PBM, on the other hand, often have a much longer wait, sometimes 14 days or more. In addition to the delays, it is clear the oncology practices have access to patient records and can more closely monitor patients which empowers them to provide the most coordinated care.
In the end, the PBMs’ lack of transparency to the patient and the general public usurps the patient’s right of choice and circumvents the prescriber’s orders and independent professional judgment.
7.1.2 Harm to Plan Sponsors
The greatest harm to plan sponsors stemming from prescription trolling and claims hijacking is increased potential for waste, particularly compared to when the claim would otherwise be filled by the community oncology practice. Many times, a community oncology practice can identify certain medications that may be difficult to tolerate or patients whose conditions may require multiple dosing refinements. In these cases, in anticipation of such modifications, practices will often dispense a 15-day supply rather than a 30- or 90-day supply. PBM specialty mail order pharmacies can lack the expertise for such forethought or do not have the experience with care management to know when a smaller supply might be the wiser, more economical choice.
Ultimately, mandatory diversion of patients to PBM mail order pharmacies leads to increased waste of often-expensive and unwanted medication, thereby increasing overall health care spending, at the expense of Medicare and taxpayers. In a study funded by the Community Pharmacy Foundation reviewing medications being returned for disposal and destruction, it was found that prescriptions originating through mail order were far more likely to have excessive amounts of unused medication remaining (i.e., 80% or more of the prescribed quantity) when compared to retail pharmacies. In the cancer space, these issues of waste can be extremely costly. ln a particularly well-documented instance, a battling advanced colorectal cancer was told that his health plan would only cover his prescription for oral oncolytics if he obtained them through the PBM’s mail-order pharmacy. After he waited nearly two weeks to receive his prescription, when it finally came, it included incorrect dosing instructions, and he was told by the PBM-owned pharmacy to send back the medication (worth $20,000) so it could be destroyed. Even when the medication was ordered again, it came with fewer pills than were prescribed. While the PBM-owned or affiliated pharmacies continue to make errors and cause patients to endure life-threatening delays, the plan sponsors – like employers and Medicaid programs – are left footing the bill for these wasted products to the tune of tens of thousands of dollars in this one instance alone.
7.1.3 Harm to Providers
In addition to circumventing the prescriber’s orders and independent professional judgment, the PBMs’ tactics of prescription trolling further serves to push the burden of performing the initial administrative functions on to the community oncology practices, while removing any attendant benefits, as the first fill is the most expensive claim. The first fills of a prescription are typically a pharmacy’s most expensive claims due to several factors, including coordination with prescriber, prior authorization efforts, researching and liaising with patient assistance programs, engaging in patient training and providing skilled nursing administration. And further, at its core, through these claim rejections, the PBMs are once again depriving providers of any ongoing and expected future business relationships with patients who initially sought to fill prescriptions with their provider.
Apart from just the lost revenue, at their core, these tactics create a lot more work for already burdened community oncology practices and make patient treatment much more difficult. In the course of the PBMs’ efforts jockeying for control of the prescription, staff at community oncology practices spends hours on the phone with all the disconnected and disjointed stakeholders, just trying to get the prescription filled and in the patient’s hands. This includes speaking with the PBM, then the insurance company, then the PBM-owned or affiliated pharmacy, then the PBM again – and this all assumes everything goes “smoothly.” It is well-documented that these additional layers of unnecessary administrative complexity burden the health care system, with health care stakeholders spending about $496 billion on billing and insurance-related costs each year. These additional administrative burdens have been found to have a direct negative impact on patient care.
Yet PBMs remained focused on maximizing profits. As the chart below show, immense profit comes along with diverting prescriptions to PBM-owned pharmacies. Within the Florida Medicaid program, the overwhelming majority of “profits” earned from dispensing brand name drugs (including cancer medications) was retained by just three PBM-owned or affiliated pharmacies.

The combination of restricted networks, prescription trolling, and the mandating of dispensation of specialty drugs at specific pharmacies has been a boon to the specialty pharmacy arms of the nation’s largest insurers and PBMs, driving disproportionate profit to them vis-à-vis their unaffiliated pharmacy peers.
7.2 What Does the Law Say?
In addition to federal and state Any Willing Provider and Freedom of Patient Choice laws, which are certainly implicated by PBMs directing patients to their wholly-owned or affiliated pharmacies and excluding community oncology practices (see, Section 6, supra), several other federal and state laws bear on the tactic of prescription trolling. First and foremost, this activity runs afoul of the Health Insurance Portability and Accountability Act and the regulations promulgated thereunder (HIPAA), which limit the disclosure of PHI by covered entities, including pharmacies and PBMs, without patient authorization. In the absence of a valid authorization, disclosures of PHI may only be made for purposes of treatment, payment, or health care operations of the covered entity. As such, a PBM’s access to and use of PHI to steer patients toward the PBM’s wholly-owned or affiliated pharmacy is a breach of HIPAA, and compromises the privacy and security of patients’ personal information. HIPAA provides, in addition to substantial civil penalties, criminal sanctions for the use of PHI in this way, which demonstrates the significance of maintaining patient privacy.
In addition, these practices likely violate many states’ Anti-Patient Steering Laws which prohibit PBM or insurer-owned or affiliated pharmacies from “steering” profitable prescriptions to their own affiliated PBM and insurance pharmacies. For example, Louisiana provides that a PBM shall not directly or indirectly engage in patient steering to a pharmacy in which the PBM maintains an ownership interest or control without making a written disclosure and receiving acknowledgment from the patient; and the PBM is further prohibited from retaliation or further attempts to influence the patient, or treat the patient or the patient’s claim any differently if the patient chooses to use the alternate pharmacy. Likewise, New Jersey makes it unlawful for a pharmacist to enter into an arrangement with a health care practitioner who is licensed to issue prescriptions, or any institution, facility, or entity that provides health care services, for the purpose of directing or diverting patients to or from a specified pharmacy or restraining in any way a patient’s freedom of choice to select a pharmacy. When the PBM engages in these underhanded tactics, it is not only directly steering the patient to a particular pharmacy without their knowledge or consent, but forcing the community oncology practice to go along with the scheme, by consenting to transfer the prescription.
Lastly, even beyond state laws, prescription trolling may impinge on other federal requirements, including Section 2 of the Sherman Act (i.e., attempted monopolization using their role and leverage as PBM gatekeeper to divert business to the PBM-owned or affiliated pharmacy), and the Employee Retirement Income Security Act of 1974 (ERISA) and its requirements that fiduciaries discharge their duties with respect to the plan solely in the interest of the participants and beneficiaries (misappropriate PHI for pecuniary gain certainly could arise to the breach of a fiduciary duty for PBMs).
The overarching legal principles are potentially tempered somewhat by recent case law involving PBM appropriation of claims data. In Trone Health Servs., Inc. v. Express Scripts Holding Co., No. 4:18-CV-467 RLW, 2019 WL 1207866, (E.D. Mo. Mar. 14, 2019), a retail pharmacy brought claims against Express Scripts, alleging Unfair Competition, breaches of contract, breaches of the implied covenant of good faith and fair dealing, interference with economic advantage, violation of uniform trade secrets act and fraud for the practice of “slamming,” that is, collecting claims information received by the PBM at the point-of-sale from retail pharmacies submitting claims for their patients, and providing that same data to Express Scripts’ wholly-owned mail order pharmacy for the purpose of soliciting the same patients to receive their prescriptions via mail order. The core of all the claims was Express Scripts’ conduct of collecting and using prescription data to boost its mail-order operations. Parsing the “black letter” language of the one-sided contract of adhesion, the Judge, however, held that the conduct was not prohibited and, in fact, was expressly allowed under the terms of the agreement with the pharmacies. While the Eighth Circuit revised the standard slightly as it relates to the pharmacy provider’s rights under HIPAA, the Court of Appeals ultimately upheld the lower court’s decision, serving as a reminder of the unbridled power that the PBMs believe themselves to hold.
7.3 What Can Be Done?
Prescription trolling and patient slamming is perhaps one of the most deceitful of the PBM tactics and requires a response at many levels to end it once and for all:
- Legislative
-
- Congress should enact federal legislation which would protect patient choice of pharmacy and prohibit PBMs from requiring patients to use the mail order and specialty pharmacies they own, creating a conflict of interest, or exploiting private patient data for those purposes.
- State lawmakers should enact anti-steering laws like Louisiana’s or Georgia’s, which prohibit PBMs from directly or indirectly steering patients to a pharmacy in which the PBM maintains an ownership interest or control.
- Regulatory
-
- The Office of Civil Rights (OCR) should pursue complaints against PBMs and PBM-owned pharmacies for misappropriation of PHI for pecuniary gain and seek fines as well as injunctive relief.
- State Boards of Pharmacy should pursue complaints against PBMs and PBM-owned pharmacies for violations of Pharmacy Practice Acts, including anti-patient steering laws.
- State Departments of Insurance should pursue complaints against PBMs and health insurers for violations of Any Willing Provider laws, stemming from efforts to deny patients the right to receive care at the pharmacy provider of their choice.
- Plan Sponsor Action
-
- Plan sponsors should negotiate PBM contract terms to require adherence to state laws and CMS guidance.
- Plan sponsors should demand that protections be given for physician-dispensed oncology medications.
Low-Ball Reimbursement
Low-ball reimbursement – when PBMs reimburse providers less than the cost of the drug – is yet another tactic taken by PBMs to effectively exclude community oncology practices, in order to retain and ensure a higher market share for the specialty drug market for their fully owned specialty pharmacies. Also known as “below water” or “underwater” reimbursement, PBMs intentionally lowball the reimbursement rates offered in one-sided, take-it-or-leave-it agreements with providers. No negotiation is offered. The ultimate goal of low-ball reimbursement is to allow the PBM to have it both ways: nominally “comply” with Any Willing Provider laws by “offering” open participation in the network, but in reality, effectively excluding pharmacy providers by pushing them to reject these unsustainable reimbursement rates, thereby diverting more patients to their wholly-owned or affiliated specialty pharmacies. While guised as a cost saving measure, PBMs actually profit off the low-ball reimbursements. As complex, multifaceted health care entities, PBMs are able to recoup any losses that might be incurred at the dispensing level by charging plan sponsors more money through spread pricing (see, Section 4, supra) or receiving rebates or other “fees” from manufacturers at the PBM level (see, Section 3, supra).
This recently played out in the wake of the collaboration agreement between Prime Therapeutics and Express Scripts, causing low-ball, below water reimbursement for community oncology practices. On April 1, 2020, Prime Therapeutics began applying Express Scripts’ lower reimbursement rates and pharmacies have been receiving abhorrently low, even negative, reimbursements. Claims specifically for lifesaving medications and limited distribution drugs are rendered below water. Notably, in June 2020, Blue Cross Blue Shield of Alabama (recognizing that these rates may not be sustainable) began increasing rates to independent pharmacies in Alabama for Blue Cross Blue Shield Alabama plans (however, this plan was the exception to the rule). Many community oncology practices continue to face unsustainable, below cost reimbursement, which is only exacerbated when taking into account direct costs associated with pharmacy operations (such as salaries and benefits of pharmacy staff, accreditation fees, shipping, dispensing fees, supplies and equipment, license fee, pharmacy dispensing software fees and adherence and symptom management software fee, postage, etc.), and indirect overhead (including rent, utilities and telephone charges).
With the impact that this has across the industry, a question is often asked: how are PBMs able to do this? The answer is simple: their excessive market power enables them to unilaterally dictate reimbursement rates where pharmacy providers have essentially no choice but to accept them. As noted above (see, Section 3, supra), over 80% of the covered lives in the United States are controlled by just five PBMs. In some markets, a single PBM could cover over 85% of the patients seen by a community oncology practice. As a result of this concentration, and the inability of patients to freely select their PBM (see, Section 3, supra), being in network with each PBM network is critical.

8.1 Who is Impacted?
Ultimately, the substantial and unreasonable reduction in reimbursements creates a provider “desert,” making it impossible for them to stay in business because market share is shifted to PBMs. This turns patients into “hot potatoes” who are passed between different providers because no provider wants to fill medications at losses of hundreds of dollars, with scant guarantee of whether any of these downward prices are actually being passed on to plan sponsors. As vertically integrated models enable PBMs to dominate the pharmaceutical supply chain, community oncology practices are often forced to accept reimbursement below cost because patients have no other choice but to participate in a plan that chooses to use one of these PBMs to manage its pharmacy benefit. Ultimately, low-ball reimbursement harms the provider of choice for the patient, which in turns harms the well-being of patients.
8.1.1 Harm to Patients
As a result of low-ball reimbursements, patients are often forced to receive care only from pharmacy providers owned by or affiliated with PBMs, replete with conflicts of interest between patient care and costs of service. This has had disastrous consequences.
For one, it is well-established that provider participation in pharmacy networks will be decreased as a result of low-ball reimbursement, leaving patients with fewer choices for care. This, in turn, will lead to worse overall care (see, Section 6, supra).
Worse yet, this has the possibility of turning patients into “hot potatoes,” where even contracted specialty pharmacies (including ones owned by or affiliated with PBMs) refuse to fill a patient’s prescription and risk losing money. Sadly, this was the experience of many patients in the immediate wake of the Express Scripts-Prime Therapeutics collaboration. In one particular example involving a Blue Cross Blue Shield of Alabama beneficiary (whose benefits processed under Prime Therapeutics), a provider attempted to fill a prescription for one of its patients but was unable to because of the unsustainable loss the below water reimbursement would have. Consequently, the provider had to attempt to transfer the patient’s prescription to at least four different specialty pharmacies (including several PBM-owned or affiliated pharmacies), in order to finally find a pharmacy that was able to fill the medication (i.e., had access to the limited distribution drug), was contracted with the payer to be reimbursed for the prescription (i.e., held the Blue Cross Blue Shield Alabama Oncology Specialty Network contract), and was willing to accept the reimbursement (i.e., take a substantial loss on the prescription). After trying multiple pharmacies in four states, the patient was finally able to get their medication from a specialty pharmacy located several states away. The whole process took almost two weeks to fill the medication for the patient, causing the patient to run out of her life-saving medication.
These low-ball reimbursement practices have not been limited to commercial plans. As yet another example of patients being “hot potatoes” with no regard for their well-being, within the TRICARE program, which was established by statute to provide health benefits coverage to active duty and retired military service members and their dependents, community oncology practices have reported per-fill losses of $500.00 on every prescription for Imbruvica (an oral oncolytic used to treat certain lymphomas and leukemias), $525.00 on every prescription for Jafaki (a common oral oncolytic used to treat certain bone marrow disorders), and $740.00 on every prescription for Alecensa (an oral oncolytic used to treat lung cancer). Community oncology practices have reported that over eighty percent of their TRICARE claims reimburse at or below cost, while those that reimburse above cost generally have a margin of less than one percent. As a result, this has caused veterans to become “hot potatoes” passed between pharmacy providers (even by PBM-owned or affiliated pharmacies), who are unwilling to fill the medication at a loss.
8.1.2 Harm to Plan Sponsors
As noted, any so-called benefits or savings are nebulous at best. In reality, vertically-integrated PBMs are able to take a “loss” at the pharmacy level, and make up for it by overcharging the plan sponsor. The anticompetitive nature of low-ball reimbursements further allows PBMs to receive “off invoice” discounts and manufacturer payments that help offset the low and under water reimbursement rates at the pharmacy level. For example, PBM-owned or affiliated pharmacies can be willing to nominally “accept” the same reimbursement terms applicable to other pharmacy providers, but they are able to recoup those “losses” by either obtaining discounts from the manufacturer in drug purchases (which are not passed through to the plan sponsor), or simply utilizing spread pricing which is where the PBM charges the plan sponsor an amount much higher than what is paid to the provider and pocketing the profits, or the “spread,” for itself (see, Section 10, infra). In a recent examples, patients and providers have studied Explanations of Benefits (EOBs) and identified instances where a PBM or health insurance company issued, in essence, two separate EOBs for the same claim: one to the provider and one to the patient. The EOBs transmitted to the provider showed the actual amounts being paid, while the one to the patient made it appear as though a much larger amount was being paid by the plan sponsor to the provider. In reality, PBM was simply keeping the difference. Thus, PBMs are using the plan sponsor’s money to profit from driving independent pharmacy providers out of the marketplace. Ultimately, the fact that plan sponsors will not experience increased savings will lead to fewer pharmacy providers in the network, making it more difficult for plan sponsors to get fair terms in the future.
8.1.3 Harm to Providers
The harm of low-ball reimbursement to community oncology practices is self-evident. Each day, more and more community pharmacy providers go out of business due to negative margins as a result of reimbursements below the acquisition and dispensing costs of the prescriptions they provide to patients. Providers often times are not able to pick and choose which rates they will accept and which ones they will not. As a result, if providers challenge low-ball reimbursement at the initial contracting stage, PBMs will likely exclude the provider from the network. For community oncology practices, that means they would be unable to dispense oral chemotherapy to patients. Likewise, when providers have raised concerns about unsustainable reimbursement rates after agreeing to participate, they risk being immediately and summarily terminated without cause.
For practices that choose to stay and accept the low-ball reimbursement rates, they experience a reduction in the ability to provide enhanced services and coordinate patient care, as a direct result of the underwater reimbursements. And when combined with the heightened credentialing standards necessary to even seek admission to these networks, providers face a veritable Catch-22 of having to choose between undertaking the high costs and extra workload of becoming accredited in order to participate in the network, only to then become unable to afford to perform the required services because of low reimbursement once admitted.
8.2 What Does the Law Say?
As in the case of restrictive networks and unreasonable barriers of entry (see, Section 6, supra), federal and state Any Willing Provider laws can offer protection against low-ball reimbursement to the extent they require PBMs to offer participation on “reasonable” and “relevant” terms and conditions. In this regard, as it relates to the federal Any Willing Provider law, CMS expressly recognized that unreasonably low reimbursement terms, which would include below water reimbursements, violate the federal Any Willing Provider law. This serves as a strong rebuke to low-ball reimbursement in the Medicare Part D space.
Recognizing this as a growing problem in the private commercial insurance sector, many states have passed “Fair Price Laws.” For example, the recently enacted New Jersey law, codified at N.J.S.A. 17b:27f-1 to -10, provide PBM pricing transparency and strengthen the rights of pharmacies to contest below-cost reimbursement. Likewise, Arkansas law prohibits PBMs from setting the price for certain generic medications below available pharmacy acquisition costs.
Several unfair trade and unfair competition laws may also be implicated by a PBM’s conduct of setting below water reimbursement to increase market share for its wholly-owned or affiliated specialty pharmacy. For example, under California’s Unfair Competition Law (UCL), Section 1702 of the California Business and Professions Code, known as the “Unfair Competition Law” or “UCL,” “any person who engages, has engaged, or proposes to engage in unfair competition may be enjoined in any court of competent jurisdiction.”
Finally, to the extent such PBM’s low-ball reimbursement is deemed to be seeking monopolization, Section II of the Sherman Antitrust Act may be implicated as well. The Sherman Act provides that it is unlawful to “monopolize, or attempt to monopolize … any part of the trade or commerce among the several states, or with foreign nations.” And further, in the context of state-level UCL claims, conduct may also be deemed to be “unfair” under the UCL if it is “conduct that threatens an incipient violation of an antitrust law, or violates the policy or spirit of one of those laws because its effects are comparable to or the same as a violation of the law, or otherwise significantly threatens or harms competition.”
8.3 What Can Be Done?
Low-ball reimbursement has the potential to fundamentally and irreparably impact our health care system for years to come, and requires action at many levels:
- Legislative
-
- Congress should enact federal legislation extending Medicare’s Any Willing Provider requirements to the TRICARE program, requiring that terms and conditions be reasonable and relevant, and allow for private enforcement of these requirements.
- States should enact Any Willing Provider Laws (where none currently exist) or amend existing Any Willing Provider laws to require that health insurance companies and PBMs allow all pharmacy providers (including community oncology practices) the right to participate in pharmacy networks based on “reasonable and relevant” terms and conditions, applicable to other similarly situated participating providers.
- States should enact laws, like New Jersey’s Fair Price law, requiring PBM pricing transparency and prohibiting below-cost reimbursement to pharmacies.
- Regulatory
-
- CMS should pursue complaints against Part D plan sponsors and contracted PBMs for unreasonably low reimbursement in violation of the federal Any Willing Provider Law and the Medicare Part D Drug Benefit Manual, seeking fines, Warning Letters, and injunctive relief.
- CMS should issue regulation providing “guard rails” on what constitutes reasonable and relevant terms and conditions, and clarify that whether given terms are “reasonable” or “relevant” can be adjudicated in a private contractual dispute between Part D plan sponsors/PBMs and pharmacies.
- State Departments of Insurance should pursue complaints against PBMs and health insurers for violations of Any Willing Provider laws, stemming from efforts to constructively deny providers the right to participate in pharmacy networks based on unreasonably low, below cost reimbursement rates.
Mandatory White Bagging for Cancer Medications
A growing – and extremely concerning – trend that has emerged is the concept of mandatory “white bagging” of oncology medications that are administered in-office by community oncology practices.
“White bagging” occurs where a physician writes and orders a particular medication for an in-office procedure, and rather than being sourced from the physician’s medication inventory, a separate specialty pharmacy fills a prescription, and delivers the drug directly to the prescriber or clinic who retains the medication until the patient arrives at their office for administration.
Likewise, “brown bagging,” which is less common, involves a similar concept, except that instead of causing the prescription to be delivered directly to the community oncology practice, the specialty pharmacy dispenses the medication to the patient him or herself, who then brings the medications into their physicians’ offices for administration in those settings.
In seeming unison, several health insurance companies (who coincidentally have integrated PBMs and specialty pharmacies) have begun to mandate that certain intravenous (IV) medications that were previously purchased by practices and administered in-office to patients, are now requiring that they be filled by the PBM-owned or affiliated specialty pharmacy through white or brown bagging. These are medications that historically have been administered in-office by community oncology practices and billed to patients’ medical benefit (as opposed to their pharmacy benefit). Because these are IV medications, they cannot be self-administered by the patient, and still need to be infused by a health care provider. In essence, these payers (which include Anthem Blue Cross of California, Blue Cross Blue Shield of Tennessee, and Cigna) have mandated that cancer patients receive their chemotherapy through white or brown bagging, to be supplied by the payers’ affiliated specialty pharmacy.
Each of these scenarios present immense concerns for patients, plan sponsors and providers alike. Community oncology practices note that white or brown bagging disrupts the chain of control of expensive cancer drugs; risking improper storage and handling of toxic substances; can unnecessarily cause delays in the onset of treatment; create waste when dosages are changed to, for example, manage adverse events; and places an administrative and liability burden on both patients with cancer and their oncologists.
9.1 Who is Impacted?
9.1.1 Harm to Patients
Patients stand to suffer the greatest as a result of payer and PBM mandatory white or brown bagging policies. Unlike instances where the community oncology practice sources the medication from its own inventory, the physician has no control over the sourcing, storage, preparation, or handling of the specialty oncology medications in white or brown bagging situations, and as a result, patients are exposed to potentially serious harm. The community oncology practice cannot guarantee the integrity and legitimacy of the products being provided by the PBM-owned or affiliated pharmacy, especially as it relates to the shipment and delivery from the specialty pharmacy to the practice. “The difficulties that white bagging policies place on cancer patients are a prime example of the potential harm.”
When medications do not follow the typical chain of custody, the integrity and safety of the medication cannot be guaranteed. When a community oncology practice sources a medication from its wholesaler to be infused in a patient, the community oncology provider is given a Transaction Report or “T3” that details every single transaction involving that medication, going all the way up to the manufacturer that made it. This ensures proper pedigree at each stage along the way. When the practice receives the drug as a white bag from a PBM-owned specialty pharmacy, it is not provided with that information. Worse yet, it has no control or insight into how the specialty pharmacy is handling that product, or how it ensured stability and integrity during the delivery process. This provides risks for patients receiving medications of unknown integrity, where chain of custody cannot be guaranteed.
Patients also stand to be impacted by excessive delays and unnecessary burdens from white bagging when forced to receive their cancer and related treatments from PBM-owned or affiliated pharmacies (as compared to when the community oncology practice sources products from its own inventory for in-office administration). Delays in receiving the medication past an anticipated date are commonly caused by a variety of factors, including failed delivery, incorrect medications being delivered, medications shipped to the wrong address, prior authorization issues, out of stock medications, etc. When medications are sourced from the community oncology practice, issues such as drug shortages can be identified right away, and adjustments made. Requiring that the prescription be sent to and filled by a PBM-owned or affiliated specialty pharmacy can cause confusion and the potential for missed treatment doses.
Finally, patients may be subject to higher out-of-pocket liability when prescriptions are “white bagged” for in-office administration. In addition to having to pay the copayment or coinsurance for the administration procedure, patients will also be responsible for a separate copayment from the pharmacy associated with the dispensed drug product. Required use of the PBM-owned or affiliated specialty pharmacy means that “reimbursement comes not from a patient’s medical benefit but from the pharmacy benefit, and that can mean higher out-of-pocket costs for patients,” as pharmacy benefit copays are typically higher than copays under the medical benefit. Moreover, because PBM-owned or affiliated pharmacies will require patients to have paid for drugs before they are shipped, this can interrupt critical treatment if patients cannot afford to pay for the therapies (a problem that is only exacerbated if the PBM-owned or affiliated pharmacy does not assist the patient in qualifying for payment assistance programs to help meet their cost-sharing obligations, which few do).
Alternatively, even when everything goes “smoothly,” waste can result if extenuating life circumstances cause a treatment plan to be adjusted or an appointment to be rescheduled and the pre-provided “white bagged” medication will not still be good by the time the appointment is rescheduled. This would not occur if the community oncology practice were able to simply source the medication from its own inventory at the time of the patient’s visit.
9.1.2 Harm to Plan Sponsors
The greatest harm to health care payers stemming from mandatory white bagging is in the form of excess drug waste. When a physician utilizes drugs the community oncology practice has on hand in its inventory, the physician is able to quickly and efficiently address patient care real time and avoid waste. Oncology regimens are complex and often require dosing adjustments at the time of administration or therapy cancellation depending on the patient’s laboratory results, scans, and other clinical considerations, such as shifts in the patient’s weight. When utilizing medications from the onsite inventory, physicians are able to make these changes at the time of administration without any delays or risk of waste (they can simply select a different medication or dose off the shelf). However, the same cannot be said if the medications are supplied by PBM-owned or affiliated specialty pharmacies.
Under white bagging mandates, the physician is required to write a “prescription” and send it to the PBM’s wholly-owned or affiliated specialty pharmacy to be filled. Circumstances requiring dosing adjustments or therapy cancellation could occur in the time between when an “order” is written by the physician, and when the medication is received from a specialty pharmacy. Moreover, once the prescription has a patient-specific label, it cannot be returned to stock, unlike products kept within the practice’s inventory for in-office administration. As a result, the entire medication would essentially go to waste, costing the plan sponsor and patient potentially thousands of dollars.
Moreover, plan sponsors face a great risk of being double billed when PBM-owned or affiliated pharmacies bill separately for the drug product, while community oncology practices bill for the procedures and supplies associated with in-office administration. When a community oncology practice submits a claim to an insurer for in-office administration of a drug to its patient, it typically submits a CPT Code for the professional services associated with the administration (e.g., CPT 96413), as well as a J-Code for the medication (e.g., J9271 in the case of Keytruda). CPT Code 96413 corresponds with “Chemotherapy administration, intravenous infusion technique, up to one hour, single or initial substance.” Thus, when submitting claims in this manner, the physician receives his or her fee for the professional services associated with mixing the drug and administering it to the patient but is also reimbursed for the costs of the medication, the diluents, the supplies, the tubing, as well as the associated overhead.
At the same time, when the PBM-owned or affiliated specialty pharmacy uses an NDC number to bill the patient’s PBM, the pharmacy may also be billing (and receiving reimbursement) for overlapping products/services (which it is not actually providing or performing). Many PBM contracts prohibit pharmacies from dispensing medications in their unfinished form, and prohibit billing medications that require reconstitution (e.g., injectable medications) as compounds (suggesting that reimbursement for the diluent and other supplies necessary for administration are included within the total payment).
In addition, many PBMs pay a “dispensing fee” on all claims in addition to the reimbursement for the drug, which is intended to cover costs that are incurred at the point of sale in excess of the ingredient cost of the drug, including the “measurement or mixing of the drug,” “filling the container,” physically providing the completed prescription to the patient, “delivery,” “special packaging,” “salaries of [workers],” “costs associated with maintaining the [ ] facility and acquiring and maintaining technology and equipment necessary to operate the [ ] facility.” While the wholly-owned or affiliated specialty pharmacy that is white bagging will be selecting the product, processing the claim, and causing delivery to the practice, many of these items for which the wholly-owned or affiliated specialty pharmacy will be receiving reimbursement are actually tasks that will ultimately be completed by the community oncology practice. The community oncology practice will continue to be responsible for mixing the drug, procuring the diluent and other necessary supplies, and physically administering the medication to the patient. Thus, this has the risk of the wholly-owned or affiliated specialty pharmacy being paid by the patient’s PBM for the same services that are also being reimbursed by the plan sponsor to the community oncology practice (and which in fact are being performed and provided by the practice).
9.1.3 Harm to Providers
Finally, the greatest harm to community oncology practices stemming from mandated white bagging are increased, unfunded administrative burdens, along with increased legal liability which the providers have no choice but to accept. Community oncology practices are faced with increased administrative burdens as they are expected to undertake all work associated with preparing, diluting, and administering the drug, without being able to seek reimbursement for the medication itself. When medications are white bagged, they typically come in the original manufacturer vials. Apart from the added burdens of storing the products and maintaining them in a separate inventory (since they are patient-specific), in order to be administered to the patient, the products must also be mixed by the practice’s staff and placed into a bag to be infused intravenously. In many instances, IV chemotherapy products are combined with other drug products, as physicians often order a “cocktail” of different drugs and therapies that must be taken in concert. Community oncology practices have to perform these services, despite the fact that they are not being reimbursed for the drug itself. This burden is only exacerbated when the physician makes changes or amendments to the treatment, often after the prescription has been written, but closer in time to when the patient is receiving care. Because the prescription has already been filled and provided by the specialty pharmacy, the practice’s staff must engage in extra work to remedy the problem.
In addition, and more concerningly, community oncology practices face additional liability for their part in prescribing and administering drugs received from outside pharmacies. In October 2012, 64 people died and over 700 people became sick as a result of contaminated compounded steroid injections supplied by New England Compounding Center (NECC). The medications had been ordered by physicians for in-office administration to their patients in clinics and surgery centers. However, due to insanitary conditions at the pharmacy, several batches of the medications had become tainted with fungus, causing many patients to develop fungal meningitis and become seriously ill or die. In the wake of this, dozens of lawsuits (including multiple class actions) were filed against not only the pharmacy, but also the clinics, surgery centers and underlying physicians. Under current white bagging mandates, community oncology practices are forced to accept this additional risk and exposure, as “the primary onus for patient safety remains with providers despite [PBMs and] health plans stripping those providers of their control over the quality and handling of drug therapies.” With white bagging, practices no longer control the acquisition of these medications, and as drug therapies become more complex, thereby requiring additional resources and focus in storing, mixing, compounding and administering the products, they are bearing an inappropriate share of the risks.
9.2 What Does the Law Say?
In April 2018, the National Association of Boards of Pharmacy issued a report entitled “White and Brown Bagging: Emerging Practices, Emerging Regulation”. The report concluded that while “the terms and conditions of this business model are most often set by third-party payers”, issues regarding authenticity and integrity of the drug and adverse patient outcomes are left to the state boards of pharmacy to grapple with in an effort to protect the public. As such, some state boards (e.g., Massachusetts) have specifically prohibited these practices, under various provisions such as “re-dispensing of medication” or handling hazardous drugs.
On the state level, several state legislatures have either prohibited or allowed white and brown bagging practices. For example, Texas, Minnesota, and New York (Medicaid) have prohibited one or both of these practices. Other states like California, have laws that require health plans to demonstrate that their medical decisions are “unhindered by fiscal and administrative management.”
At the same time, many states’ laws may bear directly on arrangements mandating that community oncology practices write prescriptions and send them to PBM-designated specialty pharmacies. For example, many states have “Anti-Patient Steering” laws, which generally prohibit health care providers from agreeing to prescriptions to a particular pharmacy. As an example, New Jersey law provides that “[i]t shall be unlawful for a pharmacist to enter into an arrangement with a health care practitioner, or any institution, facility or entity that provides health care services, for the purposes of directing or diverting patients to or from a specified pharmacy or restraining in any way a patient’s freedom of choice to select a pharmacy.” As another example, Georgia law likewise specifically prohibits pharmacies from presenting (and prohibits pharmacy benefits managers from paying) claims for reimbursement that were received pursuant to a referral from an affiliated PBM.
9.3 What Can Be Done?
Mandatory white bagging harms both patients and plans sponsors, while increasing liability to community oncology practices, and requires a response at many levels:
- Legislative
-
- States should enact laws prohibiting payer-mandated white bagging for community oncology practices and allow patients to receive their in-office oncology medications from their treating oncologist.
- Regulatory
-
- State Boards of Pharmacy should adopt regulations requiring pharmacies that fill prescriptions for white bagging obtain written consent from the physician’s office prior to dispensing the medication, and have policies and procedures in place that (i) track and assure security and accuracy of delivery for dispensed prescriptions until they are administered to the patient; (ii) provide for counseling to patients who are administered white bagged products; (iii) address the return of any prescription medications not delivered or administered to the patient; (iv) assure the confidentiality of patient information; (v) obtain consent from the patient for using such a delivery process through white bagging; and (vi) provide lowest number of vials wherever possible, so as to avoid excess closed-system-transfer requirements and potential USP <800> exposures.
- Practical Considerations
-
- Pharmacies providing white bagged medication should be required to assume all liability associated with the applicable medications/prescriptions and defend/indemnify health care providers who accept white bagged medications.
- Plan Sponsor Action
-
- Plan sponsors should demand that health plans allow patients to continue to receive administered IV chemotherapy medication provided by their community oncology practice of choice.
Spread Pricing and Middleman Profits
Spread pricing occurs when PBMs charge plan sponsors one price for the cost of a patient’s drug, while on the other side of the transaction, reimbursing the dispensing community oncology practice or pharmacy at a lower rate, while pocketing the difference, or the “spread,” for themselves. It is the classic case of the middleman mark up, but played out in a massive and extraordinarily opaque scale. This practice has recently come to light in the Medicaid context, where PBMs manage benefits for state Medicaid MCOs, and where state governments have uncovered immense spreads in drug claims for Medicaid beneficiaries. Ultimately, spread pricing practices reveal how PBMs are vertically integrated enterprises that control vast swathes of the drug supply chain create an anti-competitive marketplace, ultimately driving up the cost of drugs to public health programs and, ultimately, to patients themselves.

10.1 Who is Impacted?
10.1.1 Harm to Patients
Spread pricing harms patients by increasing premiums and drug prices. As with many other PBM pricing strategies, spread pricing has the perverse tendency to drive drug prices up as the higher the overall drug cost is, the greater opportunity for the PBM to earn a larger spread. In addition, because pricing strategies put in place by PBMs that are not equitable or uniform across different drugs, and perverse financial incentives can be created, putting patients at risk of having pharmacy providers prioritize certain patients with certain disease states over others based on the arbitrary profitability that a PBM applies to the therapy. Finally, in the context of generic drugs, where patients expect to realize the greatest pricing relief, spread pricing artificially increases the cost of such drugs, thus negating such price relief.
10.1.2 Harm to Plan Sponsors
Plan sponsors, and in particular, state Medicaid programs, have been immensely harmed in the inflated prices they – and ultimately the taxpayers – have paid to PBMs because of spread pricing. Ohio was one of the first states to audit PBMs after a Columbus Dispatch exposé revealed the extent of spread pricing in the state’s Medicaid program. Shortly after the news broke, the Ohio Department of Medicaid released a summary of its spread pricing analysis which showed PBMs grabbing $223.7 million in hidden pricing spreads within the Medicaid managed care program from Q2 2017 to Q1 2018, accounting for 8.8% of overall (pre-rebate) spending on prescription drugs.
The Ohio revelations have led to other states and the federal government investigating spread pricing practices within their states, as well as independent efforts. State government work in Kentucky, Georgia, Virginia, and Maryland has definitively quantified spread in their states’ Medicaid programs, while 3Axis Advisors – an independent pharmaceutical policy think tank – has uncovered evidence of spread pricing in New York, Illinois, Michigan and, notably, a 200-page report on spread pricing in the Florida Medicaid program.

10.1.3 Harm to Providers
Finally, spread pricing has a direct impact on providers, who rely on adequate reimbursement to serve Medicaid patients. In Ohio, the same state to expose $223.7 million in excess charges through spread pricing, many independent pharmacies were reporting such severe loses on Medicaid prescriptions that it made it virtually impossible to continue to participate in the program. The exposure of these abuses led Ohio Medicaid to require certain PBMs, including CVS Caremark, to increase the amount of reimbursements being paid to independent providers (who up until that point, were pocketing the immense spreads).
10.2 What Does the Law Say?
Given the perverse impact of spread pricing upon patients, payers, and providers, CMS’ Medicaid and Children’s Health Insurance Program (CHIP) managed care’s final rule adopted standards for the calculation of Medical Loss Ratios (MLRs). More specifically, the final rule clarified that spread pricing must be reported and included in the calculation of MLRs, which represents the percent of premium revenue that goes toward actual claims and activities that improve health care quality, as opposed to administrative costs and profits. CMS regulations require Medicaid and CHIP managed care plans to report a MLR and use and MLR target of 85 percent in developing rates.
A number of states have implemented measures to prevent PBMs from utilizing spread pricing schemes when contracting with state Medicaid managed care plans. For example, Ohio Medicaid directed its five managed care plans to terminate contracts with PBMs with spread pricing model and enter into new contracts with PBMs with transparent “pass-through” model in 2018. In similar vein, Nevada has enacted transparency bill specifying that a PBM has a fiduciary duty to a third party that contracts with the PBM for pharmacy benefit management services and must notify the third party in writing of any activity, policy, or practice of the PBM that creates a conflict of interest that interferes with the PBM’s ability to discharge its fiduciary duty. New York is also planning to no longer use PBMs and instead, to use fee for service to pay for its prescription drugs.
10.3 What Can Be Done?
The practice of spread pricing by PBMs has recently become an area of focus for plan sponsors seeking to reign in PBM abuses and reduce costs. Potential solutions to spread pricing include:
- Legislative
-
- Congress should enact federal legislation that would require pass-through pricing for covered outpatient drug prescriptions in Medicare Part D and in Medicaid (including managed care).
- States should enact laws like the Nevada law requiring PBMs to be fiduciaries to plan sponsors (i.e., PBMs must act in the plan sponsors’ interests) and providing plan sponsors with a cause of action against PBMs if they utilize opaque pricing not in the plan sponsors’ best interest or favor the PBMs’ wholly-owned or affiliated pharmacies over independent pharmacies or community oncology practices, if this would ultimately be detrimental to the plan sponsors.
- State should enact laws requiring PBMs to report drug costs charged to and paid by plan sponsors and disclosure of such reports to providers.
- Regulatory
-
- Like in Ohio, state regulators should take immediate action, where such action is permitted under enabling statutes, to prevent state Medicaid plans from contracting with PBMs using spread pricing methodology.
- The FTC should enhance oversight and revise antitrust guidance defining impermissible vertical integration structures which could, at the very least, curb the most blatant PBM anti-competitive behavior.
- Plan Sponsor Action
-
- Plan sponsors should implement robust Request for Proposal procedure to select transparent PBMs.
- Plan sponsors should review and negotiate transparent contract terms including, without limitation, an exclusive pricing benchmark.
- Plan sponsors should require PBMs to provide reporting of reimbursements paid to the pharmacies on pharmacy claims and the corresponding charges made to the plan sponsor.
Copay Accumulators and Maximizers
The increased prevalence of high deductible health plans or plans involving patient coinsurance has left more and more Americans finding themselves with significant annual out-of-pocket copayments, coinsurance obligations or deductibles for their medications. Many patients struggle to meet their deductible and pay the copays for the high-cost drugs they need to treat serious, sometimes life-threatening, illnesses like cancer. In a study published in the Journal of Clinical Oncology, the researchers found that drug abandonment and adherence problems are increasingly prevalent in patients prescribed an oral cancer medication due to higher out-of-pocket costs. To help offset these costs – especially in the oncology space, where copayments can range in the thousands of dollars – many drug manufacturers have created copay discount cards to reduce the net out-of-pocket amount to a figure that is affordable to many patients.
However, beginning in 2018, several large insurance companies and PBMs began to implement a nefarious new set of schemes called “copay accumulator programs.” Copay accumulator programs restrict manufacturer contributions to copay discount cards from being applied to patients’ annual deductibles and out-of-pocket maximums. Normally, the contributions from the drug manufacturer’s copay card would not only help offset the patient’s copay at the point-of-sale but would count toward fulfilling the patient’s out-of-pocket obligations (i.e., the deductible). Thus, after several fills of a high-cost specialty medication, the deductible would be exhausted, and the patient’s out-of-pocket would be lowered to an affordable amount. This is important, because many drug manufacturers’ copay coupon programs have annual limits or caps, preventing patients from receiving unlimited copayment assistance. Without copay accumulator programs, patients are able to afford their prescriptions throughout the whole year.
Conversely, when a copay accumulator program is implemented, the amounts of the patient’s copay that have been funded by a drug company (through a copay coupon program) no longer count towards the patient’s out-of-pocket limits. The result is that, after the patient exhausts the benefits from the manufacturer’s copay coupon program, the patient is still left with excessively high copayment obligations.
The financial impact of copay accumulator programs is demonstrated well in an example. Consider an example where a patient is prescribed a drug that costs $36,000 per year, or $3,000 per month. The patient obtains a copay coupon card from the drug’s manufacturer, with an assistance limit of $12,000 per year. The patient’s benefit plan has a $3,000 deductible and, after the deductible has been met, a monthly copay of $500. Without the copay accumulator program, the drug manufacturer would cover the $3,000 deductible in month one (January), and $500 per month each month thereafter. The patient would never run out of benefits under the copay coupon program, and would never be saddled with excessive out-of-pocket costs, significantly reducing the risk of therapy abandonment.
With the copay accumulator program in place, however, the patient would use the copay coupon to cover the monthly drug costs in months one through four (i.e., January through April), and would have no out-of-pocket expenses during those first four months of the year. However, because the copay accumulator program would prevent the amounts received through the coupon from applying toward cost-sharing requirements, the patient would still be required to pay the full deductible amount ($3,000) in month five (May), and monthly copays of $500 per month thereafter. In essence, the maximum benefits under the copay coupon program would have been exhausted at the end of April (having funded $3,000 per month). Here, when the patient is now saddled with a $3,000 bill to continue therapy he or she has been on for four months, there is tremendous risk of therapy abandonment.
These programs have been called a variety of things by different entities, including “Out-of-Pocket Protection Programs” (Express Scripts), “True Accumulation” (CVS Caremark), and “Coupon Adjustment: Benefit Plan Protection Program” (UnitedHealthcare). However, the main thrust has been to place financial roadblock in the way of patients receiving necessary care, with dubious savings being realized by plan sponsors.
Another related concept that has emerged in response to the negative patient impact from accumulators is that of “copay maximizer programs.” Like copay accumulator programs, copay maximizer programs are designed to allow payers to “extract the full value of the manufacturer’s copay support,” but in reality, swap the “financial cliff” that the patients face under accumulator programs, in favor of a slow and steady drain of resources, without any marked benefits to the patient.
For example, assume again a situation where a patient is prescribed a drug that costs $36,000 per year, and there is a manufacturer-sponsored copay coupon with an assistance limit of $12,000 per year (or $1,000 per month). In the context of a copay maximizer, the patient will still have a deductible of $3,000, but instead of a standard copay, the plan will set the monthly copay to slightly more than the coupon’s value, to, say, $1,200 per month. Each month, the patient will be responsible for $200 out-of-pocket (the difference between what is covered by the copay coupon and the set copay amount).
Worse yet, to the extent maximizer programs do actually deliver copay savings to the patient, it invariably comes with underhanded restrictions, obligating the patient to obtain the prescription exclusively from the PBM-owned or affiliated pharmacy, and allowing PBM subsidiaries to reap additional revenue. PBMs have created “secretive and independent private companies” to operate these specialty drug maximizer programs, who sometimes take fees equal to 25% of the manufacturer’s copay support program.
In each of these scenarios, however, the patient is either forced to go over the “financial cliff” in the middle of the year (when their copays skyrocket) and risk drug abandonment, or is forced to utilize a PBM-owned or affiliated pharmacy with limited real financial benefits (or face exorbitant out-of-pocket costs).
11.1 Who is Impacted?
Copay accumulator and maximizer programs have clear negative impact on all stakeholders.
11.1.1 Harm to Patients
The harm of copay accumulators and maximizer programs is felt most acutely by patients – especially cancer patients. Unlike instances where there might be lower cost generics available to be used as alternatives when a brand manufacturer’s copay coupon benefits expire, there are no alternatives for the high-priced oncology medications, and when manufacturer copay coupon programs run out as a result of copay accumulator programs, “the individuals who need assistance the most will be unable to receive it, and will end up paying more for their treatments.”
“This poses an adverse impact on adherence to medication regimens, especially when a support mechanism is not in place.” Studies have shown that patients impacted by copay accumulator programs fill their prescription 1.5 fewer times than patients who are not impacted. More critically, data has shown that patients impacted by copay accumulator programs have experienced a 13% drop in adherence – that is, they’ve fallen off therapy – between months 3 and month 4 of a plan year (coinciding with when they reach the annual cap for manufacturer-sponsored copay coupon programs). This is significant as over 75% of impacted patients have said that their adherence will suffer as a result of these programs.
These findings and observations of direct patient harm have been backed up by literature. In a study published in the American Journal of Managed Care, the authors found that after the implementation of copay accumulator programs, Health Savings Account patients on certain high-cost specialty drugs had “significantly lower monthly fill rates, higher risk of discontinuation, and lower [percentage of days covered],” suggesting that copay accumulator programs have “the potential to negatively affect specialty drug use.” This rings true in the cancer context as well. According to a study in the Journal of Clinical Oncology, nearly half of patients with cancer abandon their prescriptions when out-of-pocket costs reach $2,000. Nonadherence can have dire consequences to patients, and accounts for 10% of hospitalizations and 125,000 deaths each year.
Perhaps the best evidence of patient harm is the stories from the patients themselves. In one instance, a nurse case manager from Ohio with multiple sclerosis had long managed her disease with medications, and was able to afford them through copay coupon programs. However, in May 2018, she discovered that her health plan had instated a copay accumulator program, that required her to pay $3,600 per month for her prescription drugs until she met an $8,800 deductible, forcing her to consider rationing her medication that allowed her to function in her daily life. In another well-publicized incident, a 27-year-old hemophilia patient had been able to afford the $38,000 for his maintenance drugs with the assistance of manufacturer copay coupon programs. However, once his health plan instituted a copay accumulator program, he was unable to afford the $6,350 deductible. As a result of his immediate and unforeseen inability to afford the medications, he was left with untreated bleeds, resulting in internal bleeding, and needing additional surgeries to correct. “The patient has been in and out of the hospital, is currently in a wheelchair, and is not working, all at a cost of $3.5 million.”
One of perhaps the most sinister aspects of copay accumulator and maximizer programs for patients is the overall lack of transparency. These programs lack any semblance of transparency, and are “often implemented without a patient’s knowledge or full understanding of their new ‘benefit.’”
Ultimately, because patient receiving medications that have lower-cost generic products have the ability to switch to such generic products in the face of copay accumulator and maximizer programs, it is the sickest patients requiring the highest-priced drugs that are most egregiously affected by these programs, and are in essence “subsidizing the patients who are adequately served by lower-cost pharmaceuticals that have low or no copays.”
11.1.2 Harm to Plan Sponsors
When the PBMs created and rolled out copay accumulator programs, they were billed as a cost savings tool for plans sponsors, such as employers. In theory, it does make sense when applied to high-cost branded medications, when a lower-cost, equally effective generic product is available. These programs counteract manufacturer efforts to retain market share for brand drugs once generics have become available, and further the interests of pushing patients to lower cost alternatives. However, in the oncology space, cancer care is for life saving treatment and does not have the same risks of “overutilization,” nor are there cheaper alternatives available.
Instead, the result of copay accumulator or maximizer programs is that the harms and additional costs to plan sponsors caused by drug abandonment and non-adherence will far outweigh any potential savings to be gained from them. From increased hospitalizations, additional treatments, and more catastrophic care, it is well-established that plan sponsors save money when patients stay adherent to the drugs they are prescribed. This is especially true in the cancer context, where studies have suggested that the increased plan costs caused by non-adherence due to copay accumulator programs was more than double than that of all other disease groups.
Worse yet, many employers and plan sponsors do not even know what they are getting or whether such programs have been instituted. While nearly 20% of commercial medical insurance policies sold in 2018 will have copay accumulator/maximizer programs built in, “most employers who have purchased/are purchasing these plans are unaware these programs are present in the coverage” and “have no idea how it will adversely affect their employees’ care.” This is especially alarming considering the secretive operations of copay maximizer programs, where the prescription is typically required to be filled at the PBM-owned or affiliated pharmacy, and related or affiliated companies take up to 25% of the copayment assistance made available by the manufacturer.
For example, with Express Scripts’ SaveonSP program, a commercial plan sponsor declares specialty drugs to be “non-essential health benefits,” making them covered by the plan, but not subject to out-of-pocket maximums mandated by the Affordable Care Act. In turn, the patients’ out-of-pocket costs are set to the maximum annual value of a manufacturer’s copay coupon program. “For instance, a program with a total value of $20,000 in copayment support would require a patient to pay $20,000 annually for their drugs, without regard to the plan’s out-of-pocket maximums.” Thereafter, to avoid these inflated costs, the beneficiaries must enroll separately in the SaveonSP program, and have their prescriptions filled exclusively by Express Scripts’ Accredo specialty pharmacy. SaveonSP then charges a fee equal to 25% of the copayment support, or $5,000 in the above example. This is in addition to the profit generated by Express Scripts’ wholly-owned pharmacy by filling the prescription.
Ultimately, while copay accumulator and maximizer programs might seem like a good short term solution, the devil is in the details, and in reality, these programs will ultimately increase costs for plan sponsors in the long run, including increased hospitalizations, additional care, and overall increases to drug prices.
11.1.3 Harm to Providers
Finally, community oncology practices are harmed by manipulative copay accumulator and maximizer practices as well. When physicians prescribe a particular oncology treatment to be dispensed out of the community oncology practice, they undertake a “difficulty and time-consuming process” involved in finding financial assistance for their patients. This includes finding manufacturer-sponsored copay coupon programs, providing resources to patients, and potentially providing supporting documentation to these programs. The copay accumulator and maximizer programs will add additional complexities in the patient coverage process and will only increase “the administrative burden on practice staff, who will now need to understand the nuances of co-pay accumulators and maximizers; as well as help explain to patients why some of the assistance is not helping them to reach their deductible.”
In addition, community oncology practices are further impacted when their patients discontinue prescribed therapy due to cost. Many community oncology practices are contracted with payers under value-based arrangements, where they take responsibility – and sometimes risk – for the outcomes of patients. If a patient stops taking his or her therapy once the copay coupon program is exhausted, that patient may wind up in the hospital or needing additional care. This will in turn negatively impact community oncology practices’ performance under value-based contracts.
11.2 What Does the Law Say?
Federal statutory law is silent on the issue of copay accumulator and maximizer programs.
However, in 2019, HHS finalized the Notice of Benefit and Payment Parameters for 2020 (NBPP 2020), which only allowed health plans to implement copay accumulator programs when both a brand and generic medication were available. In essence, this would have allowed plans to steer patients to less costly, generic medications when possible, but would provide protections for patients – including cancer patients – who did not have access to alternative, less costly medications.
However, on May 7, 2020, HHS released its Notice of Benefit and Payment Parameters for 2021 Final Rule, which clarified certain confusion created by different agencies’ guidance, and now allows health plans to implement copay accumulator programs regardless of whether or not a generic alternative is available. When patients cannot afford their medications, they may rely on copay assistance (i.e., coupon cards from drug manufacturers). These coupon cards not only contribute toward the patient’s copay but also count toward the patient’s annual deductible. Thus, as of July 30, 2020, HHS has not only allowed health plans to implement these programs but has removed key protections for cancer patients.
Fortunately, however, several states have enacted their own laws governing copay accumulators (importantly, in NBPP 2021, HHS explicitly stated that the Final Rule does not preempt state laws that govern the use of copay accumulator programs in state-regulated health plans). At this time, four states (Illinois, West Virginia, Virginia, and Arizona) have enacted copay accumulator legislation. While these apply to state-regulated plans (and not to Exchange-based health plans), they provide protection against certain PBM conduct.
For example, Virginia Statute § 38.2-3407.20 requires health plans to include any amount paid by or on behalf of a plan enrollee when calculating an enrollee’s overall contribution to any out-of-pocket maximum or any cost-sharing requirement to the extent permitted by federal law and regulation. Likewise, in Illinois, 215 Ill. Comp. Stat. Ann. 134/30 requires health plans to apply any contributions (i.e., third-party payments, financial assistance, discount, product vouchers, or any other reduction in out-of-pocket expenses) for prescription drugs made by or on behalf of an enrollee toward that person’s deductible, copay, or cost-sharing responsibility, or out-of-pocket maximum.
An additional eight states have some form of legislation pending to address copay accumulator/maximizer programs.
11.3 What Can Be Done?
- Legislative
- State should enact laws that require health plans to include any amount paid by or on behalf of a plan enrollee when calculating an enrollee’s overall contribution to any out-of-pocket maximum or any cost-sharing requirement.
- Regulatory
-
- HHS should rescind the Notice of Benefit and Payment Parameters for 2021 Final Rule, and institute Notice of Benefit and Payment Parameters that, at the very least, reinstates, strengthens, and clarifies the protections for patients receiving medications without lower cost alternatives.
- Plan Sponsor Action
-
- Plan sponsors should inquire with PBM whether copay accumulator and/or maximizer programs are being employed, and demand that protections be given for oncology medications that lack lower cost alternatives.
Maximum Allowable Cost (MAC) Pricing
Maximum Allowable Cost pricing, or “MAC,” is one of the most significant and challenging issues facing independent pharmacies throughout the United States today. While not as impactful to community oncology practices providing cancer care as many of the other topics addressed in this exposé, MAC has nevertheless become one of the most manipulated and opaque methods by which PBMs control reimbursement to independent pharmacies and has become a catalyst for legislative efforts to rein in PBM conduct.
MAC is typically defined as the maximum amount of money that PBM will pay a pharmacy for certain multi-source drugs, typically multi-source generic drugs. It is well established that generic drugs make up the vast majority of drugs dispensed throughout the United States. For example, according to the Association for Accessible Medicines, generic drugs account for approximately 89% of all prescription drugs dispensed in the United States. Thus, generic drugs constitute the majority of drugs dispensed to patients throughout the United States meaning MAC pricing present a significant issue as it pertains to provider reimbursement.
MAC began as a mechanism to save money in health care and incentivize selective and intelligent purchasing practices, but MAC has since evolved over time into a PBM tool that can be manipulated by PBMs to increase revenues in several different ways. MAC pricing is a PBM created pricing benchmark – MAC prices and MAC lists are prepared exclusively by PBMs and considered by the PBMs to be proprietary and confidential. Moreover, PBM-set MAC rates need not have any relationship to a drug’s market clearing acquisition cost. As such, the creation and publication of PBM MAC prices and MAC lists are shielded from the public and avoid public scrutiny.
PBMs’ ability to keep MAC lists and MAC prices from the public has enabled PBMs to utilize MAC pricing to increase their revenues and to effectuate certain PBM practices that lead to higher revenues, including the PBM practice of spread pricing, wherein a PBM reimburses a pharmacy provider one price for a drug but collects a higher amount from the plan sponsor and retains the difference. The fact that MAC pricing is shrouded in secrecy, and there is no requirement for MAC rates to have any basis in real costs, creates substantial profit opportunities for PBMs and has resulted in substantial challenges for independent pharmacy providers over the past several years.

12.1 Who is Impacted?
PBMs’ secretive MAC pricing tactics have caused harm to payers, providers, and most critically to patients.
12.1.1 Harm to Patients
The improper use of MAC pricing tactics harm patients throughout the United States by limiting patient care access – this specific issue is on display in the case Rutledge v. PCMA which successfully went before the Supreme Court of the United States in December of 2020. In Rutledge, Arkansas enacted a law, Act 900, with the purpose of addressing this patient-based issue, which was especially pronounced in rural areas. MAC pricing appears to often disproportionately harm patients in rural areas, who often do not have access to a broad catalogue of different (sometimes cheaper) products, thereby harming these patients specifically. “MAC methodologies are resulting in pharmacies closing down, especially in rural areas . . . [and] approximately 44% of Arkansans live in rural areas.” The potential for patient harm based upon improper pricing and reimbursement tactics, including MAC pricing combined with spread pricing was also discussed at length in the Pennsylvania Auditor General’s Report on PBMs, wherein it was noted that “small pharmacies often see the most vulnerable patients . . . [a]nd if small pharmacies are forced out of business, these patients will have to travel greater distances to get the medications they need[.]” Thus, there is ample objective evidence that PBMs’ MAC pricing tactics are causing harm to patient populations throughout the United States and that this harm may be particularly pronounced in rural settings.
12.1.2 Harm to Plan Sponsors
In addition to harming patients, improper MAC pricing tactics by PBMs also potentially harm all payers including Medicare, Medicaid, employers, and taxpayers, although studies indicate this may be particularly pronounced in the Medicaid context. “PBMs’ control of MAC definitions allows them to manipulate the MAC concept in whatever ways they choose.” Thus, MAC lists do not afford payers and sponsors with the ability to have predictability or in any way guarantee savings but instead give PBMs unfettered discretion to control precisely which drugs are on a particular MAC list and to ensure only those drugs which they are making money on remain on the list and those which they are not are removed from the list. In assessing potential harm of PBMs’ MAC pricing tactics, it is important to note that MAC pricing applies to drugs, most commonly generics, and not to specific programs (e.g., Medicaid. The implication is that improper MAC pricing tactics can affect all payers, including federal and state governments, and by extension, taxpayers.
As mentioned, MAC pricing is one of the primary methods by which PBM spread pricing is effectuated, wherein the PBM bills a plan sponsor one price and reimburses the pharmacy provider a lower amount. Several studies have shown that improper MAC pricing tactics, in connection with spread pricing, has been prominent in the Medicaid context, including in Florida, Georgia, Illinois, Kentucky, Maryland, Michigan, New York, Ohio, and Virginia. Pennsylvania’s report on PBMs noted that in 2017 “three PBMs made between $2 million and nearly $40 million on spread pricing, earning average profits between 28 cents and almost $13 per Medicaid prescription filled.
In order to implement spread pricing via MAC pricing, PBMs create numerous different MAC lists including separate MAC lists for each individual payer as well as separate MAC lists for PBM network providers which enables a PBM to bill a plan sponsor one rate but pay the pharmacy a separate and frequently a much lower rate. Florida, Michigan, New York, and Ohio are three states that exemplify the harm caused by PBM MAC pricing tactics to state specific Medicaid programs.
12.1.3 Harm to Providers
Finally, improper MAC pricing tactics by PBMs also harm providers due to unsustainable reimbursement by PBMs. In Rutledge, the District Court acknowledged that numerous pharmacies had been harmed by these tactics and were closing down, noting that “[i]ndependent community pharmacies have had to eliminate employees during the last five to ten years due to the financial hardships they have faced.” The court further noted that “[i]ndependent community pharmacies in Arkansas are in economic distress.”
These unreasonably low MAC prices are further exacerbated by the fact that PBMs are often slow to make price adjustments to MAC drugs when there are market conditions that would result in the PBM reimbursing a higher amount (i.e., an increase in acquisition cost), but relatively quick to make price adjustments based on market conditions that would result in the PBM reimbursing a lesser amount (i.e., a decrease in acquisition cost).
12.2 What Does the Law Say?
Given its prevalence in commercial insurance contracts and Medicaid programs, MAC pricing has largely been regulated by the states. Currently, 36 states have some form of MAC law or MAC appeal law in place. While the different state laws vary in the level of protections they afford to pharmacies regarding MAC, there are several general characteristics in these state MAC laws. Typically, robust MAC laws will establish criteria for placing a drug on a MAC list, establish an appeal process for challenging questionable MAC pricing, and set requirements for updating MAC lists. Texas and Georgia are example of such laws. In Texas, a PBM may not include a drug on a MAC list unless: (1) the drug: (A) has an “A” or “B” rating in the most recent version of the United States FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, also known as the Orange Book; or (B) is rated “NR” or “NA” or has a similar rating by a nationally recognized reference; and (2) the drug is: (A) generally available for purchase by pharmacists and pharmacies in [Texas] from a national or regional wholesaler; and (B) not obsolete. Further, the PBM must develop a process for pharmacies to appeal MAC prices of a drug on or before the 10th day after the claim is submitted and the PBM must respond within 10 days.
Texas’ MAC appeal requirements also require that when an appeal is successful, the PBM must (1) adjust the MAC that is the subject of the appeal effective on the day after the date the appeal is decided; (2) apply the adjusted MAC price to all similarly situated pharmacies as determined by the PBM; and (3) allow the pharmacy that succeeded in the appeal to reverse and rebill the pharmacy benefit claim giving rise to the appeal. When appeals are not successful, the PBM must identify and disclose (1) each reason the appeal was denied; and (2) the NDC number from the national/regional wholesalers from which the drug is generally available for purchase by pharmacies in Texas at the MAC price that is the subject of the appeal. Moreover, in Texas, there are separate guidelines governing MAC in the Medicaid context. Although there are some functions an MCO may delegate to a PBM in Texas, there are also certain functions for which an MCO is ultimately responsible despite the delegable nature of the function. These expressly include “negotiation and establishment of pharmacy provider reimbursement rates [and] cultivation and maintenance of MAC pricing lists.” Thus, certain laws in the Medicaid context potentially provide additional recourse against payers in addition to PBMs as is the case in Texas.
12.3 What Can Be Done?
Effective responses to improper MAC pricing by PBMs require action at various levels:
- Legislative
- Congress should enact legislation at the federal level prohibiting MAC manipulation including a requirement as to transparency in MAC pricing on both sides of the PBM—at the plan sponsor side as well as the provider side.
- States must take more aggressive action against PBMs’ MAC pricing tactics and enact new laws or else enhance existing laws that mandate transparency in reimbursement which should include robust laws that protect both plan sponsors and providers from manipulative MAC pricing practices, especially as it pertains to claims that are paid from taxpayer dollars, e.g., Medicare and Medicaid.
- For legislative efforts to be effective, the laws enacted must provide a deterrent beyond solely relying on government enforcement. Thus, it is imperative that states enact laws or enhance existing laws by including or adding “private rights of action” to ensure plan sponsors and providers have recourse against improper PBM MAC pricing tactics and also make violation of MAC laws by PBMs an unfair or deceptive trade practice act in accordance with existing state law.
- State laws should strive for greater uniformity, including in how MAC is defined to prevent inconsistencies in reimbursement practices throughout the country, greater/broader appeal rights (requiring that the drug utilized as the basis for the MAC rate is readily available and conforms with the state’s prescription substitution laws), and to ensure that PBMs cannot take liberties in placing drugs that do not meet a uniform definition of a MAC drug on a MAC list.
- MAC laws should permit providers to choose how MAC appeals are filed rather than permitting PBMs to force providers to use a pharmacy services administrative organization (PSAO) – this ensures that if PSAOs are not responsive to MAC issues, pharmacy providers can pursue the appeals on their own or hire third parties that may be more effective at addressing MAC issues.
- Regulatory
-
- There should be increased scrutiny over PBM MAC pricing tactics at the federal level through CMS/OIG audits.
- Increased scrutiny over PBM MAC pricing tactics should happen at the state level through audits by both the Departments of Insurance and Departments of Health and/or state Boards of Pharmacy.
- Plan Sponsor Action
-
- Plan sponsors must demand more from PBMs during the contracting process including specific information on how pharmacies are being reimbursed and the use of MAC lists as it pertains to both the plan sponsors’ relationship with the PBM as well as pharmacies’ relationships with the PBM to understand if spread pricing is being used and/or whether pharmacies are being harmed by improper MAC reimbursement.
Effective Rate Reconciliation
In yet another opaque and underhanded ploy, PBMs have created and utilized the concepts of Generic Effective Rate (GER) and Brand Effective Rate (BER) to essentially reprice drugs, and claw back pharmacy reimbursements, sometimes more than a year after drugs are dispensed. GER and BER (collectively known as the “Effective Rate”) measure the discount that the PBM contractually must deliver for its client (i.e., plan sponsors) to a benchmark called Average Wholesale Price (AWP) for generic prescription drugs and for brand-name prescription drugs, respectively. However, because they are assessed retrospectively and on a network level basis, it is tantamount to giving PBMs unbridled discretion as to how they will pay a given pharmacy, and still technically be in compliance with the reimbursement terms of the agreement.
Worse yet, PBM methods of imposing and recouping Effective Rate assessments are equally deceitful. Not only did many PBMs foist such reimbursement terms on pharmacy providers retroactively without their knowledge or consent (for example via retroactive contracts with the pharmacy providers’ PSAOs), but in many instances, the pharmacy providers only learned of the Effective Rate reconciliation when the PBMs, either directly or indirectly, simply began withholding payments due to offset the alleged Effective Rate overpayments.
Because of its after-the-fact assessment applied across an entire network of pharmacy providers, Effective Rates allow PBMs to circumvent Maximum Allowable Cost laws enacted by many states (see, Section 12, supra), and hinders pharmacy providers’ ability to challenge underwater reimbursements on generic prescriptions. At its most basic level, Effective Rate is not reflected at the point of sale and it provides an opportunity for PBMs to take back a substantial amount of reimbursements on prescription drug claims that were already dispensed to patients.
Similarly, PBMs have also created another pricing mechanism called Dispending Fee Effective Rate (DFER) to recoup dispensing fees already paid to providers that provides no purpose to reduce plan sponsors’ drug spending. DFER allows a PBM to pay one dispensing fee at the point-of-sale, and afterwards claw-back a portion of this dispensing fee down to the contractually specified DFER. This particularly pernicious type of effective rate undermines the cost-plus pass-through contracts that many state Medicaid programs are contemplating, or moving to, in response to outrage over spread pricing. DFERs could allow the PBM to pass through the state-mandated dispensing fee, only to claw it back after the fact, without the state’s knowledge.
13.1 Who is Impacted?
13.1.1 Harm to Patients
As with many other PBM tactics, including spread pricing (see, Section 10, supra), rebates (see, Section 4, supra), and DIR fees (see, Section 5, supra), Effective Rate reimbursement frameworks have the ability to increase the gross price for medications, notwithstanding a potentially lower net price. For Medicare Part D patients, Effective Rate forces them to reach “donut hole” and pushes patients into “catastrophic coverage” at a much faster rate. As discussed in detail below, this results in the patients being responsible for a greater share of the costs of the medication.
13.1.2 Harm to Plan Sponsors
While it is billed as a “cost containment” and pricing guarantee to payers, in actuality, Effective Rate reimbursement schemes do little to lower the overall costs of drugs. Effective Rate prices are invariably tied to percentage discounts off of reported AWP (as shown in the graphic on page 78, an inherently unreliable pricing benchmark), enabling PBMs to deliver on savings guarantees, while not actually lowering overall costs (as lower generic and brand-name prescription drug costs for plan sponsors would in turn lower overall revenue for PBMs).
An even more pernicious feature of Effective Rate pricing arrangements is that they provide PBMs with the ability to collect “spread” between what they charge their clients (e.g., employers and plan sponsors) and what they pay their providers (e.g., pharmacies and community oncology practices) without having to put their clients in traditional spread pricing contracts. Instead, PBMs can simply sign one contract with a client guaranteeing, say, an 82% discount to AWP and a different contract with their pharmacy network guaranteeing an 87% discount to AWP. Both contracts are highly confidential, so the “buyer” (the employer) and “seller” (the pharmacy) of drugs does not know what each other are paying/receiving. Even if the employer demands a full pass-through contract, in which no spread is taken off the claim, the PBM will simply pass-through what it charges its client to the pharmacy at the time of the transaction, and then claw the overpayment back at a later time through its effective rate adjustments. At the end of the day, in this hypothetical example the PBM has locked in 5% of AWP for its services, regardless if it collects that up front or months after the transaction.
The value of Effective Rate contracts to the PBM does not end there. That’s because for generic drugs, AWP is designed to do exactly the opposite of what prices should do over time for generic drugs – AWP is designed to increase, not decrease over time. So, in our hypothetical example, the hidden 5% of AWP locked in by the PBM becomes more and more valuable each year to the PBM as AWPs diverge from true generic acquisition costs.

13.1.3 Harm to Providers
As noted above, Effective Rate reimbursement has had an especially damaging impact on providers. By effectively circumventing MAC laws, PBMs are able to reimburse many pharmacies below water on claims, leaving them without any recourse to challenge such reimbursements through legally-mandated appeals processes. This has particularly effect on providers who only dispense a limited range of generic products, such as community oncology practices. PBMs’ reconciliation of Effective Rate is a significant financial hurdle to community oncology practices because oncologists generally treat patients with a handful of drugs compared to other community retail or chain pharmacies who have a broad and diverse patient population.
13.2 What Does the Law Say?
At the federal level, in addition to the guidance on spread pricing generally (see, Section 10, supra), GER and BER reconciliations are properly considered Direct and Indirect Remuneration (DIR), which Medicare Part D plan sponsors must report to CMS. This at least, in theory, requires PBMs and Part D plan sponsors to disclose the extent and amount of GER/BER, regardless of whether it is passed-through to the plan sponsor or retained by the PBM.
At a state level, many states have enacted laws that would prohibit these types of post-point-of-sale reconciliations and clawbacks with respect to private health plans. For example, Tennessee law provides that neither a health insurance company nor a PBM may “charge a pharmacist or a pharmacy a fee related to a claim unless it is apparent at the time of claim processing and is reported on the remittance advice of an adjudicated claim.” Likewise, Indiana law explicitly regulates the practice of “effective rate of reimbursement,” and provides that a PBM may not “[r]educe, directly or indirectly, payment to a pharmacy for pharmacist services to an effective rate of reimbursement…”
Finally, Effective Rate reconciliations may impinge on the multitude of “Prompt Payment” laws that exist in virtually every state in the country. For example, Mississippi’s Pharmacy Benefit Prompt Pay Act requires PBMs to pay electronically submitted claims in full within fifteen days. PBMs’ later-in-time retraction of the amounts paid could violate those requirements.
13.3 What Can Be Done?
Effective Rate reimbursement requires a response at many levels:
- Legislative
- States should enact laws, like Tennessee’s and Indiana’s that prohibit recoupment of fees on claims that were not reflected at the point-of-sale or otherwise ban Effective Rate reimbursement as a construct altogether.
- States should enact MAC Appeal Laws (where none exist) or amend existing MAC laws to prohibit health insurers and PBMs from circumventing MAC appeal rights through Effective Rate reimbursement constructs.
- Laws should be enacted, like New Jersey’s Fair Price law, requiring PBM pricing transparency and prohibiting below-cost reimbursement to pharmacies.
- Regulatory
-
- CMS should audit Part D plan sponsors and contracted PBMs to determine whether GER/BER is appropriately reported and reconciled to CMS at the end of each Plan Year.
- State Departments of Insurance should pursue complaints against PBMs and health insurers for violations of Any Willing Provider Laws, stemming from efforts to constructively deny providers the right to participate in pharmacy networks based on unreasonably low, below cost reimbursement rates.
- Plan Sponsor Action
-
- As part of the PBM contract, plan sponsors should require PBMs to pass through any and all amounts PBMs received from the pharmacies after the point-of-sale on a claim-by-claim level.
- As part of the PBM contract, plan sponsors should require PBMs to seek a permission prior to implementing a contracted-rate with the pharmacies (e.g., GER).
Conclusion
The list of PBM abuses and games is seemingly never-ending and evolving. But the reality is that we are only just scratching the surface of understanding what these abusive health care middlemen are doing. Simply put, PBMs have overwhelmingly abused their responsibility to protect Americans from this country’s drug pricing crisis, instead exploiting the opacity throughout the drug supply chain to enrich themselves. Their many abuses go well beyond just questionable rebate practices, and hurt patients and plan sponsors (including employers, Medicare, and Medicaid).
Unfortunately, their impact is only becoming more pronounced, especially in oncology. More and more cancer drugs are coming out in oral formulations, further shifting care away from the medical space and into the pharmacy space. These expensive therapies are very attractive to PBM’s because of the potential for high prices that yield high rebate revenues, high DIR fees, and eventually, high spreads – all of which are a function of the drug’s cost.
And even outside of the pharmacy benefits realm, through vertical integration, PBMs have been able to exert considerably more influence in the other areas, such as injectable biosimilars and intravenous chemotherapies. Not only can PBMs can leverage these for steep originator and rebates (thereby stifling the biosimilar industry for their own gain), but PBMs have instituted mandatory white bagging policies to take even in-office administration out of the hands of community oncology practices.
The bottom line is this: today’s drug supply chain is designed for cancer patients to receive inferior treatment, while paying more out-of-pocket.
The time for action to stop PBM abuses is now. Each day that goes by, community oncology patients, practices, and professionals become increasingly powerless because of horizonal PBM consolidation and vertical integration with insurers.
Fortunately, however, solutions do exist. These include legislative efforts at both the state and federal levels. Many states’ existing laws serve as prime examples of how they can be successfully implemented to protect the interests of patients and health care payers (like employers, Medicaid programs, and taxpayers). In addition, based on many laws that are currently on the books, regulators (both state and federal) have tremendous tools available to them, that up until this point, have not been widely utilized. The time is critical that regulators – including CMS, OCR, the FTC, state Boards of Pharmacy and state Departments of Insurance – take much need action to rein in the unchecked power of PBMs.
The time for sitting back and letting market forces address the issues is over. The time for action to stop PBM abuses is now.
